Ifng
-
Official Full Name
interferon, gamma -
Overview
This gene encodes a member of the type II interferon family. The protein encoded is a soluble cytokine with antiviral, immunoregulatory and anti-tumor properties and is a potent activator of macrophages. Mutations in this gene are associated with aplastic anemia.[provided by RefSeq, Nov 2009] -
Synonyms
IFNG;interferon, gamma;IFG;IFI;interferon gamma;IFN-gamma;immune interferon
Recombinant Proteins
- Human
- Ferret
- Cynomolgus
- Mouse
- Bovine
- Porcine
- Rhesus macaque
- Rat
- Rabbit
- Marmota monax (Woodchuck)
- Equine
- Pig
- Horse
- Ovine
- Chicken
- Cattle
- Dog
- Guinea pig
- Sheep
- Hamster
- Zebrafish
- Canine
- Goat
- Siniperca chuatsi (Mandarin fish)
- Golden hamster
- HEK293
- E.coli
- Yeast
- Barley Grain
- CHO
- Hordeum Vulgare
- COS-7 cells
- Mammalian Cells
- Human Cells
- C-His
- Non
- His
- Fc
- Strep II
- T7
- GST
- Flag
- Avi
- DDK
- Myc
Background

Fig1. IFN-γ-producing immune cells and key functions. (Jonathan Thuner, 2023)
What is IFNG protein?
IFNG (interferon gamma) gene is a protein coding gene which situated on the long arm of chromosome 12 at locus 12q15. This gene encodes a soluble cytokine that is a member of the type II interferon class. The encoded protein is secreted by cells of both the innate and adaptive immune systems. The active protein is a homodimer that binds to the interferon gamma receptor which triggers a cellular response to viral and microbial infections. The IFNG protein is consisted of 166 amino acids and its molecular mass is approximately 19.3 kDa.
What is the function of IFNG protein?
IFNG is an immunomodulatory protein that is primarily released by activated lymphocytes and has a variety of functions. IFN-γ can promote the antiviral ability of host cells, enhance the activity of macrophages, promote the activation of T cells and B cells, and regulate the inflammatory response, which plays an important role in immune response and anti-microbial infection. In addition, IFN-γ can inhibit cell proliferation and regulate immune tolerance and other physiological processes.
IFNG Related Signaling Pathway
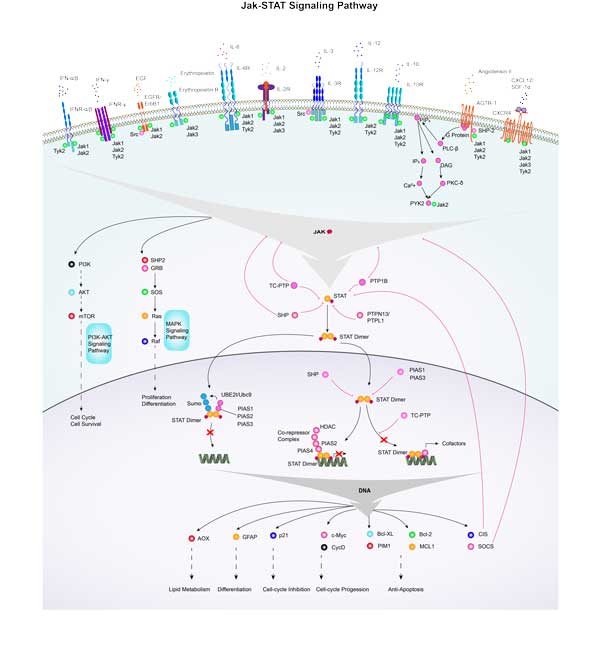
IFNG is primarily signaling through its receptor IFNGR, and when IFNG binds to the receptor, it induces dimer subunits of the receptor and activates intracellular Janus kinases (JAKs), which leads to phosphorylation of signal transduction and transcriptional activators (STATs), especially STAT1. Phosphorylated STAT1 dimerizes and is transferred into the nucleus to regulate expression of specific genes, including interferon-stimulated response element (ISRE). In addition, IFN-γ also activates other signaling pathways, such as the MAPK and PI3K/Akt pathways, which are closely related to the regulation of cell proliferation, survival, and inflammatory response.
IFNG Related Diseases
Diseases associated with this protein include immune deficiency diseases, autoimmune diseases, chronic inflammatory diseases, and certain infectious diseases. Its abnormal function or overactivation can lead to dysregulation of the immune response, triggering a variety of inflammation and diseases, including rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus and others. In the tumor microenvironment, IFNG has a complex bidirectional effect, both promoting anti-tumor immune response and possibly supporting immune evasion and tumor growth. Changes in their levels can affect the development of many cancers.

Fig2. Immune surveillance and escape from IFN-γ-dependent anticancer activity. (Chiou-Feng Lin, 2017)
Bioapplications of IFNG
Using its powerful broad-spectrum activity against viruses, bacteria and parasites, IFNG is used to treat infections including tuberculosis, chronic granulomatosis and other refractory infections. In vaccine development, IFNG can be used as a molecular adjuvant to enhance the immune response triggered by the vaccine and improve the efficiency of antigen presentation, thereby enhancing the efficacy of the vaccine.
Case Study
Case study 1: Katelyn J McCann, 2022
Interferon γ (IFNγ) is an essential and pleiotropic activator of human monocytes, but little is known about the changes in cellular metabolism required for IFNγ-induced activation. We sought to elucidate the mechanisms by which IFNγ reprograms monocyte metabolism to support its immunologic activities. IFNγ increased oxygen consumption rates (OCR) in monocytes, indicative of reactive oxygen species generation by both mitochondria and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Transcriptional profiling revealed that this oxidative phenotype was driven by IFNγ-induced reprogramming of NAD+ metabolism, which is dependent on nicotinamide phosphoribosyltransferase (NAMPT)-mediated NAD+ salvage to generate NADH and NADPH for oxidation by mitochondrial complex I and NADPH oxidase, respectively. Interestingly, inhibition of NAMPT in healthy monocytes completely abrogated the IFNγ-induced oxygen consumption, comparable to levels observed in CGD monocytes. These data identify an IFNγ-induced, NAMPT-dependent, NAD+ salvage pathway that is critical for IFNγ activation of human monocytes.

Fig1. Primary human monocytes were stimulated with media alone, LPS, IFNγ, or LPS plus IFNγ for 24 hours prior to the start of the assay, then ECAR was measured according to the Seahorse Glycolysis Stress Test.

Case study 2: Julian J Freen-van Heeren, 2020
Long-lasting CD8+ T cell responses are critical in combatting infections and tumors. The pro-inflammatory cytokine IFN-γ is a key effector molecule herein. They researchers recently showed that in murine T cells the production of IFN-γ is tightly regulated through adenylate uridylate-rich elements (AREs) that are located in the 3' untranslated region (UTR) of the Ifng mRNA molecule. Loss of AREs resulted in prolonged cytokine production in activated T cells and boosted anti-tumoral T cell responses. Here, they investigated whether these findings can be translated to primary human T cells.
Utilizing CRISPR-Cas9 technology, they deleted the ARE region from the IFNG 3' UTR in peripheral blood-derived human T cells. Loss of AREs stabilized the IFNG mRNA in T cells and supported a higher proportion of IFN-γ protein-producing T cells. Importantly, combining MART-1 T cell receptor engineering with ARE-Del gene editing showed that this was also true for antigen-specific activation of T cells. MART-1-specific ARE-Del T cells showed higher percentages of IFN-γ producing T cells in response to MART-1 expressing tumor cells.

Fig3. Representative concatenated flow cytometry dot plots of IFN-γ production of control and ARE-Del T cells after removal from stimulus for indicated time points.

Quality Guarantee
High Purity

Fig1. SDS-PAGE (IFNG-116H) (PROTOCOL for western blot)
High Bioactivity

Fig2. Activity Data. (IFNG-116H)
Involved Pathway
Ifng involved in several pathways and played different roles in them. We selected most pathways Ifng participated on our site, such as Proteasome,Cytokine-cytokine receptor interaction,HIF- signaling pathway, which may be useful for your reference. Also, other proteins which involved in the same pathway with Ifng were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Antigen processing and presentation | NFYC,PDIA3,TAPBP,CD8B1,KIR2DL1,RFXANK,HLA-A,KIR2DS2,HLA-DMA,HLA-DRA |
| Regulation of autophagy | IFNA8,IFNA5,ATG13,IFNA10,ATG16L1,GABARAPA,ULK2,INSB,ATG14,IFNA2 |
| Herpes simplex infection | DAXX,EIF2AK1,CSNK2A2,TRAF2,EEF1DB,MCRS1,TAF6,TTC6,STAT1A,HNRPK |
| Epstein-Barr virus infection | HLA-E,PSMD11,TRAF1,HSPA2,CCNA1,POLR3B,PSMC5,PLCG2,ENTPD3,MAPK9 |
| Inflammatory bowel disease (IBD) | IL21,STAT3,IL1A,HLA-DQA1,HLA-DRB5,TGFB3,H2-AB1,TGFB1,HLA-DMA,HLA-DQA2 |
| TGF-beta signaling pathway | ID2B,GDF7,RHOA,SMAD3A,SMAD5,TGFBR1B,BMPR1AB,AMHR2,BMP7B,NBL1 |
| Jak-STAT signaling pathway | GRB2B,PRP2,PTPN2A,IFNA7,IL2RGA,STAM,AOX1,LIF,HRASB,IL4R |
| HIF- signaling pathway | HIF1A,PFKL,RELA,PDHA1,PLCG1,MAPK3,MKNK2,NOX1,EP300,HK1 |
| Osteoclast differentiation | NFATC1,SFPI1,IFNGR2,FHL2,LILRA1,SOCS3,IKBKB,NCF4,NCF2,TEC |

Fig1. Diagram of pathways by which IFNγ regulates cellular NAD+ metabolism. (Katelyn J McCann, 2022)

Fig2. IFN-γ and IL-17 signaling pathways working together, separately. (Hui Dai, 2020)
Protein Function
Ifng has several biochemical functions, for example, cytokine activity,interferon-gamma receptor binding. Some of the functions are cooperated with other proteins, some of the functions could acted by Ifng itself. We selected most functions Ifng had, and list some proteins which have the same functions with Ifng. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| cytokine activity | TIMP1,TGFB1A,BMP8B,FGF2,CER1,IL12BA,IFNPHI3,CD40LG,CCL19A.1,HMGB1 |
| interferon-gamma receptor binding | DNAJA3,IFNG1-2,IFNG1-1 |
Interacting Protein
Ifng has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with Ifng here. Most of them are supplied by our site. Hope this information will be useful for your research of Ifng.
IFNGR1;b8_vaccw
Ifng Related Signal Pathway
Resources
Research Area
NeuroinflammationNatural Killer Cells (NK Cells) Markers
Inflammatory Cytokines & Chemokines
Cytokines and Growth Factors Secreted by VSMC
Conventional/Classical Dendritic Cells
Cytotoxic T Cells (CTLs)
gamma delta T Cells
Natural Killer T (NKT) Cells
Regulatory T Cells (Tregs)
T Cell Cytokine Signaling
Inflammatory Bowel Diseases Therapeutic Targets
T Follicular Helper (Tfh) Cells
Th1 Cells
Th9 Cells
MDSC Cytokines and Growth Factors
Cytokines in Tumorigenesis
Interferon
Related Services
Related Products
References
- Voigt, RM; Keshavarzian, A; et al. HIV-associated mucosal gene expression: region-specific alterations. AIDS 29:537-546(2015).
- Lv, W; Duan, QL; et al. Expression of B-cell-associated genes in peripheral blood mononuclear cells of patients with symptomatic pulmonary embolism. MOLECULAR MEDICINE REPORTS 11:2299-2305(2015).