Cell and Gene Therapy
🧪 RAD51-128H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-339 aa
🧪 RAD51-2155H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-242aa
🧪 RAD51D-2156H
Source: E. coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-253 aa

🧪 RAD51L1-2157H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-384aa
 Full L.
Full L.
🧪 RAD51-134H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 1-339 aa
🧪 TNFSF4-272H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 51-183 a.a.
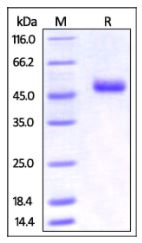
🧪 TNFSF4-490H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: Gln51-Leu183

🧪 Tnfsf4-20M
Source: HEK293
Species: Mouse
Tag: Fc
Conjugation:
Protein Length: Gln49-Leu198
🧪 Tnfsf4-21M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Gln49-Leu198
Background
Overview
- What are cells and genes?
Cells are the basic building blocks of all living things, and genes can be found deep within cells. Genes are small sections of DNA that carry genetic information and instructions for making proteins, which help build and maintain the body.
- What are genetic diseases?
Genetic diseases, also known as genetic diseases, are health problems that occur because of some abnormality in an individual's genetic material. These diseases can be caused by genetic changes (mutations) in a single gene, abnormalities in chromosomes, or complex interactions between many genes and environmental factors. Genetic diseases include the following types: monogenic diseases, chromosomal diseases, and multifactorial or complex diseases.
- The difference between cell therapy and gene therapy
Cell Therapy: Cell therapy usually involves the transplantation of autologous or allogeneic cells, which can be undifferentiated stem cells or differentiated mature cells. In the treatment of genetic diseases, cell therapy aims to improve the disease state by replacing, repairing, or enhancing damaged or abnormal cells in the patient's body.
Gene Therapy: This technique modifies a person’s genes to treat or cure disease by replacing a disease-causing gene with a healthy copy, inactivating a non-functioning gene, or introducing a new or modified gene into the body.
In all, cell and gene therapy (CGT) is an advanced medical approach that involves the use of cellular and genetic materials to treat or potentially cure diseases. CGT aims to treat diseases at the molecular or cellular level, offering the potential to restore normal function or cure diseases that were previously untreatable.
Types of CGT Products
CGT products represent a revolution in the medical field, using cells or genetic material to treat or prevent disease, with significant advantages in terms of therapeutic efficacy and mechanism of action compared to traditional medicines.

CGT Related Tools
CGT is a cutting-edge medical approach that includes multiple types of proteins and strategies to treat disease. These proteins and technologies play a key role in CGT, and their development and application offer new hope and therapeutic approaches for the treatment of genetic diseases, cancers and other serious diseases. As science advances, it is likely that more innovative proteins and strategies will join the ranks of CGT in the future. More and more are being used not only for proteins, but also for vector tools to deliver target genes.

The CRISPR-Cas System
Of all the CGT technology categories, the CRISPR-Cas system is a revolutionary gene-editing technology that uses bacteria's natural defense mechanisms to precisely modify the genome. The system consists of CRISPR sequence, Cas protein and sgRNA (single guide RNA). The CRISPR gene sequence is composed of leader, repeat and interval sequences, while the Cas gene codes for proteins that work with CRISPR sequence regions and are essential for defense processes. Several types of Cas genes, such as Cas1-Cas10, have been identified. Studies have shown the existence of multiple CRISPR/Cas systems, including class I-III and the newly discovered class IV-VI. CRISPR/Cas9 belongs to Class II, while Cpf1 (now renamed Cas12a) belongs to Class V and is one of the systems that is getting a lot of attention.
Cas proteins are key components in the CRISPR-Cas system, and there are many types of them with different structures and functions. Here are some known types of Cas proteins and related system.
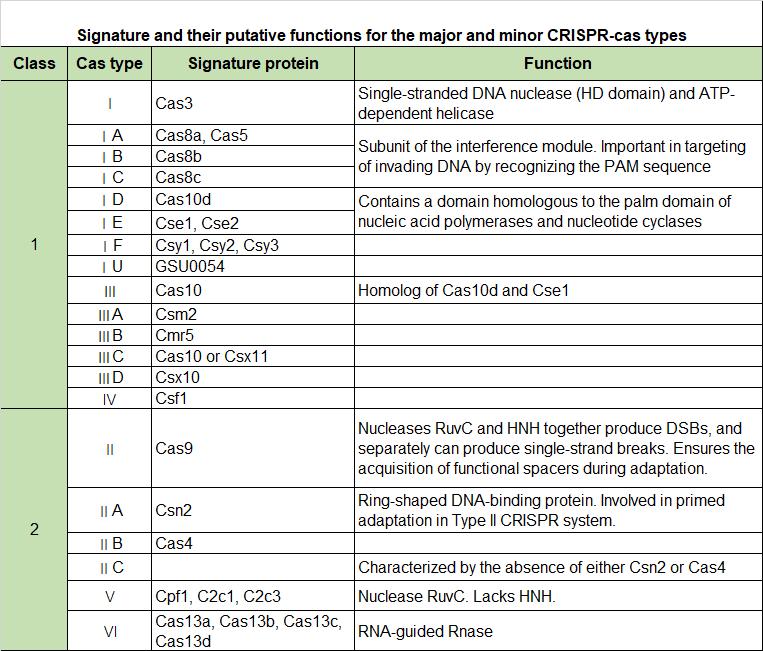
In this system, in addition to the Cas protein that plays a cutting role, other auxiliary proteins are also important, undertaking the repair, integration and even RNA editing functions of gene editing.
- Transcription Activator like Effect nuclease (TALENs): A fusion of a transcription activator (TAL) and a FokI nuclease that can specifically recognize and cut DNA.
- Zinc Finger nuclease (ZFN): The zinc finger domain is used to recognize specific DNA sequences and is combined with FokI nuclease for DNA cutting.
- RecA: A protein involved in homologous recombination that plays a role in the process of gene repair.
- Rad51: Similar to RecA, it is involved in DNA homologous recombination and repair.
- Mad7: shows similar functionality to other CRISPR-Cas systems, but is receiving increasing research attention due to its small size and high efficiency.
- Cascade Complex: A multi-protein complex in the CRISPR system that recognizes and binds to target DNA sequences.
- UvrD: It is involved in the DNA repair process in bacteria and can be used as an aid to gene editing.
- NgAgo: A nuclease found in the bacterium Akermannia (N. gora) that enables DNA editing without templates.
- Cpf4/CasY: A relatively new CRISPR analogue that enables targeted editing with single-stranded DNA.
- HinCII: This is a restriction enzyme often used in genome editing projects to cut specific sequences.
- Recombinase Enzymes (RE): such as Cre recombinase and Dre recombinase, using paired sites to perform gene recombination.
- PhiC31 Integrase: Integration of foreign DNA into certain yeast sites, often used in transgenic integration studies.
- PiggyBac Transposase: It is a transposition enzyme used to insert or remove DNA fragments in the genome.

Applications
The therapeutic approach to CGT is valued for its highly targeted and potentially one-time-cure ability, which uses cellular or genetic material to treat or prevent disease.
Genetic diseases: Treat diseases caused by mutations in a single gene, such as cystic fibrosis, sickle cell anemia, and certain inherited blindness. For example,
- Cancer treatment: The use of CAR T-cell therapy and gene editing technology to boost the immune system's attack on tumors.
- Cardiovascular disease treatment: Using stem cells to repair heart muscle tissue and improve heart function.
- Tissue repair and regenerative medicine: The use of stem cells to repair or replace damaged tissue.
- Rare diseases treatment: New treatment options for diseases for which there are no effective treatments.
- Autoimmune diseases treatment: Regulating the immune system or replacing defective cells to treat diseases.
With further research, CGT is expected to be applied to more disease areas, including cancer, cardiovascular diseases, neurological diseases, metabolic diseases and various infectious diseases. As the technology matures and is produced at scale, the cost of CGT treatment is expected to decrease, allowing more patients to benefit. The CGT drives the development of personalized medicine to provide patients with customized treatment options, while at the same time, more research is needed to ensure their safety, effectiveness and accessibility.
Case Study
Case Study 1: Recombinant Cas9 Nuclease Protein
Using CRISPR/Cas9, it is possible to target virtually any gene in any organism. A major limitation to its application in gene therapy is the size of Cas9 (>4 kb), impeding its efficient delivery via recombinant adeno-associated virus (rAAV). Therefore, researchers developed a split-Cas9 system, bypassing the packaging limit using split-inteins. Each Cas9 half was fused to the corresponding split-intein moiety and, only upon co-expression, the intein-mediated trans-splicing occurs and the full Cas9 protein is reconstituted. This study revealed that the nuclease activity of our split-intein system is comparable to wild-type Cas9, shown by a genome-integrated surrogate reporter and by targeting three different endogenous genes. An analogously designed split-Cas9D10A nickase version showed similar activity as Cas9D10A.

(Dong-Jiunn Jeffery Truong, 2015)
Fig1. Results after FACS: The importance of structural integrity of the split–intein–Cas9 system.
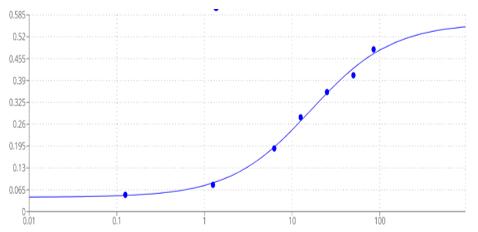
Case Study 2: Recombinant Human RAD51 (RAD51-134H)
Searching for factors to improve knockin efficiency for therapeutic applications, biotechnology, and generation of non-human primate models of disease, researchers found that the strand exchange protein RAD51 can significantly increase Cas9-mediated homozygous knockin in mouse embryos through an interhomolog repair (IHR) mechanism. IHR is a hallmark of meiosis but only occurs at low frequencies in somatic cells, and its occurrence in zygotes is controversial. Using multiple approaches, they provide evidence for an endogenous IHR mechanism in the early embryo that can be enhanced by RAD51. This process can be harnessed to generate homozygotes from wild-type zygotes using exogenous donors and to convert heterozygous alleles into homozygous alleles without exogenous templates.
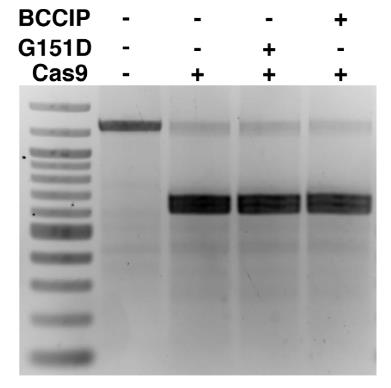
(Jonathan J Wilde, 2021)
Fig2. In vitro Cas9 nuclease activity assay incubated with RAD51.
Case Study 3: Active Recombinant Chicken IFNA (IFNA-1127C)
Covalently closed circular DNA (cccDNA) of hepadnaviruses exists as an episomal minichromosome in the nucleus of an infected hepatocyte and serves as the template for the transcription of viral mRNAs. It had been demonstrated that interferon alpha (IFN-α) treatment of hepatocytes induced a prolonged suppression of human and duck hepatitis B virus cccDNA transcription. In our efforts to identify IFN-induced cellular proteins that mediate the suppression of cccDNA transcription by the cytokine, we found that downregulating the expression of signal transducer and activator of transcription 1 (STAT1), structural maintenance of chromosomes flexible hinge domain containing 1 (SMCHD1), or promyelocytic leukemia (PML) protein increased basal level of cccDNA transcription activity and partially attenuated IFN-α suppression of cccDNA transcription. In contrast, ectopic expression of STAT1, SMCHD1, or PML significantly reduced cccDNA transcription activity. Mechanistic analyses demonstrated that STAT1, SMCHD1, and PML were recruited to cccDNA minichromosomes and phenocopied the IFN-α-induced posttranslational modifications of cccDNA-associated histones.

(Junjun Cheng, 2020)
Fig3. Treatment of dstet5-CCC cells with rChIFN-α did not alter the amount of cccDNA.
Case Study 4: Recombinant Human CD19 protein (CD19-3307HP)
Autologous chimeric antigen receptor (CAR) T cells targeting the CD19 antigen have demonstrated a high complete response rate in relapsed/refractory B-cell malignancies. However, autologous CAR T cell therapy is not an option for all patients. Here researchers optimized conditions for clinical-grade manufacturing of allogeneic CD19-CAR T cells using CD45RA-depleted donor memory T cells (Tm) for a planned clinical trial. Tm were activated using the MACS GMP T Cell TransAct reagent and transduced in the presence of LentiBOOST with a clinical-grade lentiviral vector that encodes a 2nd generation CD19-CAR with a 41BB.zeta endodomain. The resulting CD19-CAR(Mem) T cells expanded on average 34.2-fold, and mean CAR expression was 45.5%. The majority of T cells were CD4+ and had a central memory or effector memory phenotype, and retained viral specificity.

(Young-In Kim-Hoehamer, 2023)
Fig4. Percentage of CD19-CAR expression as determined by flow cytometry.
Advantages
- Wide Coverage: CGTs related proteins include cas proteins, cytokines, growth factors and so on. Nearly more than 1000 related proteins from over 10 different species.
- High Quality: We provide high quality protein products and efficient protein manufacturing techniques.
- Experienced: We have professional research team with experience of many years in the field of molecular and cell biology.
- Customized services: We offer a wide range of unique, reliable, and flexible customized services, catering to all your needs.
Related Resources
- GMP Proteins
- Targets of CAR-T Cell Therapy
- Labeled Car-T Proteins
- Cytokines for Organoid Culture
- Cytokines
- Growth Factors
- Cytokine Therapy
- RNA Viruses Triggered Signal Pathway
- Cas9 Enzyme: A Revolutionary Tool Ushering in a New Era of Life Sciences
- Traditional Gene Manipulation Technologies and Zinc Finger Nucleases (ZFNs)
- Cas9 Protein: Unveiling the "Molecular Scissors" of Gene Editing
- TALENs: Precision Gene Editing for Research & Therapeutics
- The Big Three of Gene Editing: ZFNs, TALENs & CRISPR - Understand Their Differences and How to Choose
-
Unlocking the Power of Cas9: The Future of Genetic Science
-
Unlocking CRISPR-Cas9: The Gene Editing Revolution
-
CRISPR-Cas9: Revolutionizing Genetic Science!
-
ZNFs: The Precision Tools of Gene Editing
-
Why TALENs Still Beat CRISPR for Precision Gene Editing!
-
CRISPR vs. TALEN vs. ZFNs: The Ultimate Gene Editing Battle!
References
- Cheng J.; et al. Interferon Alpha Induces Multiple Cellular Proteins That Coordinately Suppress Hepadnaviral Covalently Closed Circular DNA Transcription. J Virol. 2020 Aug 17;94(17):e00442-20.
- Wilde JJ.; et al. Efficient embryonic homozygous gene conversion via RAD51-enhanced interhomolog repair. Cell. 2021;184(12):3267-3280.e18.
- Truong DJ.; et al. Development of an intein-mediated split-Cas9 system for gene therapy. Nucleic Acids Res. 2015;43(13):6450-6458.
- Kim-Hoehamer YI.; et al. Development of a cGMP-compliant process to manufacture donor-derived, CD45RA-depleted memory CD19-CAR T cells. Gene Ther. 2023;30(3-4):222-231.







