Quality Management Systems
Certificates
Creative BioMart quality management systems are ISO certified. Combined with our digital system, raw material tracking, and large-scale facilities, Creative BioMart products are suitable for long-term scalable supply. If a Supplier Survey or Quality Statement is required, please contact us.
Quality Management Systems
The Quality Management System (QMS) is a structured system that defines the policies, procedures, responsibilities, processes, and resources for implementing and maintaining quality management in Creative BioMart. This strategic framework helps Creative BioMart ensure our products and services consistently meet customer's requirements and enhance customer satisfaction.
Quality Policy

Professional

High Quality

Innovation

Keep Improving

Communication

Customer Satisfaction

Confidentiality

After-Sales Guarantee

Safety

High Cost Performance

Time Control
Global-Oriented
Strict Quality Management System
Creative BioMart has established a comprehensive quality management system for the entire production process from raw material entry to product production, testing, warehousing, release, and delivery in accordance with ISO requirements, and has obtained certification. We control all aspects of the production process: employees, equipment, materials, methods, environment and measurements to ensure consistent, stable and repeatable results across different production processes. Through the efficient operation of this system, we ensure the management of the entire life cycle of products and services from design and development, pilot testing, formal production testing, marketing to customer feedback. The entire process maintains integrity and traceability through detailed tracking and tracing of relevant SOPs, records, plans, reports, quality standards, test reports, annual data audits, etc. Creative BioMart uses a variety of monitoring and measurement methods to identify problems in real time and make improvements to ensure the effectiveness, adequacy and applicability of our quality management system.
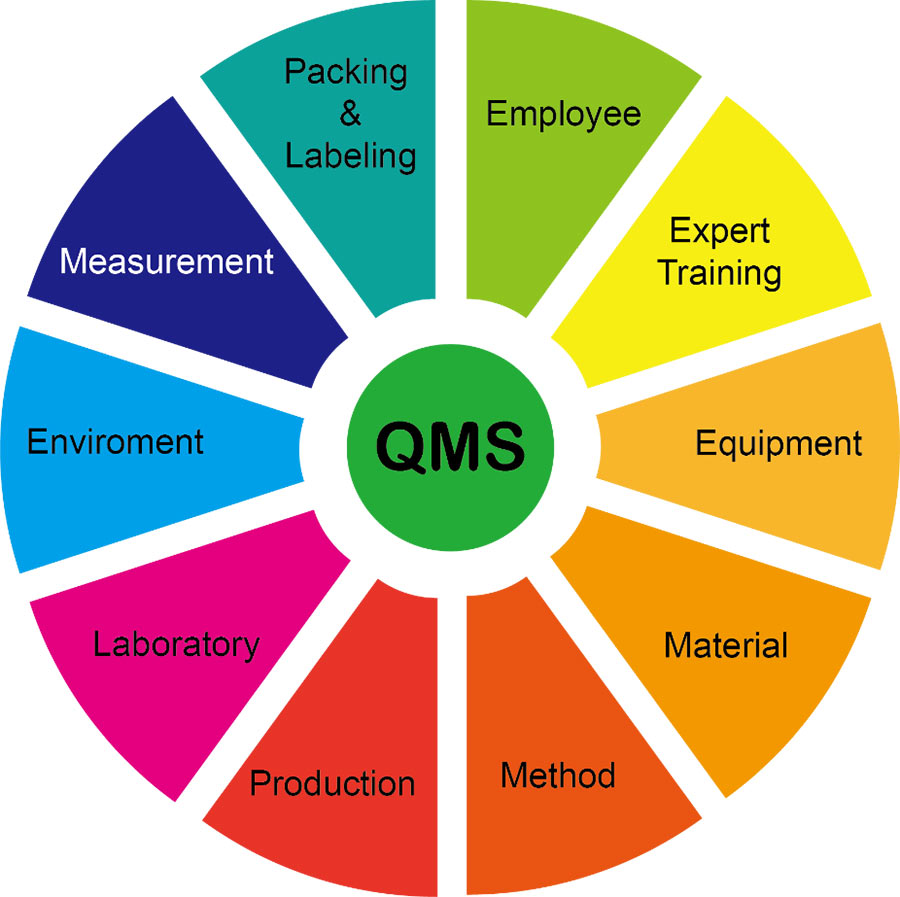
GMP Quality Management System
Good Manufacturing Practices (GMP) are a set of standard rules for personnel, sites and facilities, equipment, production management, quality management, and document management developed by the U.S. Food and Drug Administration (FDA) and adopted globally. Ensure product quality, safety and effectiveness as well as compliance throughout the production process. Creative BioMart strictly abides by the GMP specifications set by the FDA and has implemented a comprehensive GMP quality management system. It ensures that product quality meets relevant standards, and problems that arise during the production process are discovered and solved in a timely manner. This system is in line with international quality standards and covers the entire production cycle, including material management, product production and quality control.
- High purity, high biological activity
- Standardized processes and quality control
- Full range of equipment and comprehensive analytical methods
- Experienced scientists and professionals
Documents and Document Control
Proper documentation is at the heart of a well-functioning quality management system. Documentation must be developed to support the implementation, education, deployment, and control functions of the system. Creative BioMart has established a complete document management system in accordance with various quality management system specifications, including laws and regulations, operating instructions, process instructions, quality manuals, quality records, etc. In order to ensure the effective implementation of the quality management system, we will continue to supplement documented information to ensure the standardization, effectiveness and traceability of the production process.
Quality Management Process
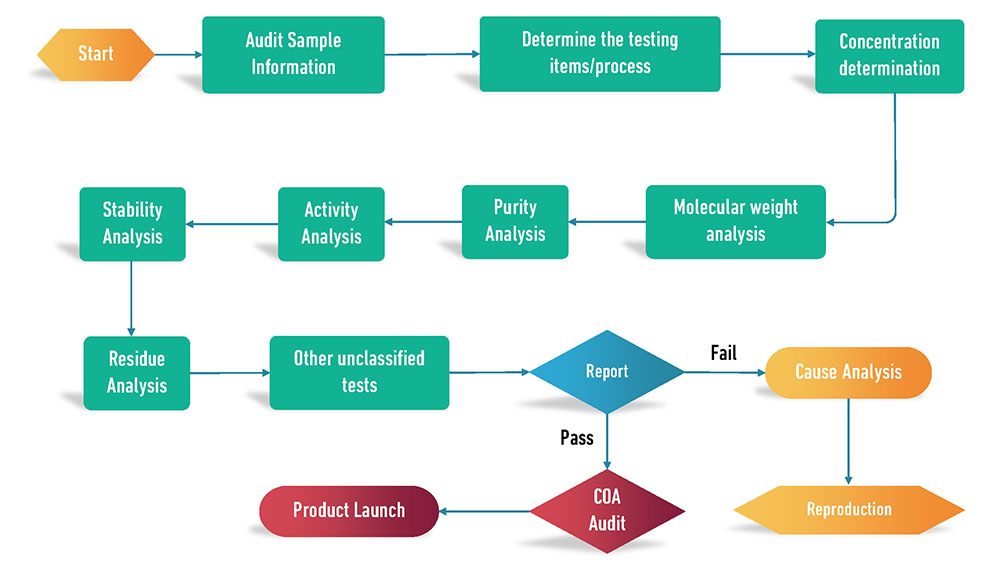
Personnel, Facility, and Equipment
Creative BioMart is a leading supplier in providing a wide range of products and services in the field of biotechnology and pharmaceutical development. Our company is headquartered in a state-of-the-art facility located in USA. The site features a modern and fully equipped laboratory equipped with cutting-edge technology and state-of-the-art equipment. Our team of highly skilled scientists and researchers consists of experts in various fields such as molecular biology, biochemistry, pharmacology, and toxicology. With a combined years of experience in the industry, our personnel are dedicated to providing top-notch products and innovative solutions to our clients.
Our laboratory is equipped with the latest equipment and technology, allowing us to perform a wide range of analysis including cell-based assays, molecular biology techniques, protein expression and purification, drug screening, and toxicology studies. Our team of scientists and researchers are capable of handling complex projects with precision and efficiency, ensuring fast turnaround times and accurate results. With a commitment to quality and compliance, we adhere to strict regulatory guidelines and maintain a high level of transparency and communication with our clients throughout the entire research process.
One of the key advantages of partnering with Creative BioMart is our unwavering commitment to delivering exceptional quality in every project we undertake. We understand the importance of producing reliable and accurate data in the field of biotechnology, where even the small errors can have significant consequences. That's why we have implemented stringent quality control measures at every stage of the research process to ensure that our clients receive results that are both scientifically robust and reproducible.
Our laboratory is designed to meet the high industry standards, with dedicated workspaces for each stage of the research process to prevent cross-contamination and ensure the integrity of our samples. We invest in the technology and equipment to support our research efforts, regularly calibrating and maintaining our instruments to guarantee the accuracy and reliability of our data. Additionally, our team undergoes regular training and certification to stay abreast of the latest advancements in the field, allowing us to provide cutting-edge solutions to our clients.
Laboratories

Production Equipment
-

Prokaryotic Culture Facility
-

20L Prokaryotic Culture Facility
-

Incubator
-

Eukaryotic Culture Facility
-

Eukaryotic Culture Facility
-

Eukaryotic Culture Facility
-

Chromatography Cabinet & Purifier
-

Ultrasonic fragmentation instrument
-

Centrifuge
-

Freeze dryer
-

Freeze dryer
-

AKTA purifier 100
Quality Control Equipment
-

Agilent 1200 series
-

Biocore T200
-

ForteBio Octet
-

DynaPro ZetaStar Light Scattering Detector
-

Multi-Angle Light Scattering (MALS) Detector
-

Spectra 200 TEM
-

Confocal Laser Scanning Microscope
-

BD FACSAria II
-

Auto Chemistry Analyzer
Quality Control
At Creative BioMart, we understand that the success of your research depends on the quality of the services you receive. That's why we go above and beyond to guarantee the quality and accuracy of our work, delivering results that you can trust and rely on. Whether you are conducting preclinical research, drug development, or biomarker discovery, Creative BioMart is your trusted partner for high-quality research and development services.

Purity and Batch Consistency
-
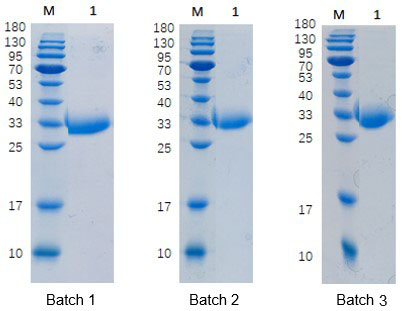
SDS-PAGE
-

HPLC
Molecular Weight
-

MALDI-TOF
-
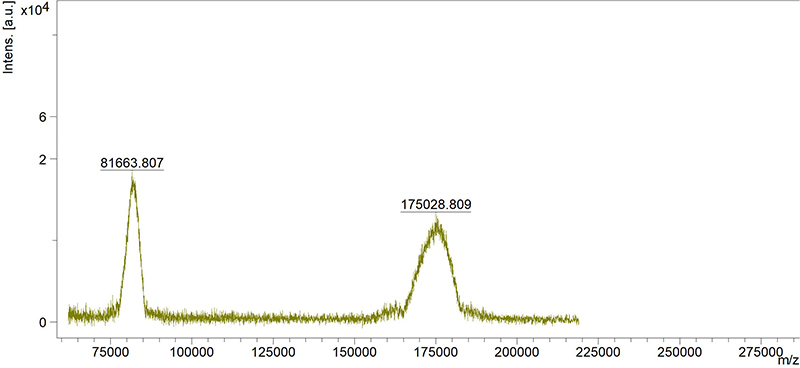
MALDI-TOF
Imaging
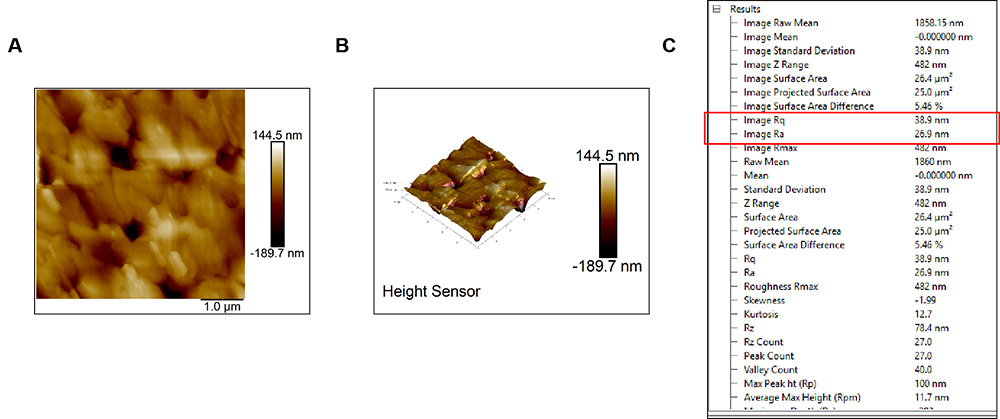
AFM results
Structural

CD results for protein secondary structure analysis
Enzyme Activity
Bioactivity-FACS

Advantages
By following QMS guidelines, Creative BioMart can demonstrate that our manufacturing processes are robust and consistent, reducing the risk of product deviations or failures. This is particularly important for our products, which are often complex molecules that may be prone to variability during production. A strong quality management system based on GMP principles helps to minimize the potential for product defects and ensures that each batch of product meets the required specifications.
In addition to GMP compliance, a quality management system also include other elements such as quality control testing, risk management, and documentation practices. Quality control testing involves the evaluation of product quality at various stages of the production process, from cell culture and expression to purification and formulation. These tests include assays to determine protein purity, identity, and potency, as well as tests for contaminants such as endotoxins or host cell proteins.
Risk management involves identifying potential risks in the production process, assessing their likelihood and impact, and implementing strategies to mitigate or eliminate these risks.
Documentation practices are also essential for maintaining a robust quality management system for our products. Creative BioMart keeps detailed records of all aspects of the production process, including batch records, testing data, deviations, and corrective actions. These records are essential for demonstrating compliance with regulatory requirements and for identifying and resolving quality issues that may arise during production.
By implementing a robust quality management system, Creative BioMart can ensure that our products are manufactured in a controlled and consistent manner, minimizing the risk of product deviations or failures. This, in turn, helps to enhance product quality, safety, and efficacy.
Improved Quality: A QMS helps improve the quality of products or services, as it focuses on consistent production and process control.
Enhanced Customer Satisfaction: Since the system is directed towards meeting customer requirements, it ultimately increases satisfaction level.
Cost Efficiency: Reducing waste and improving operational efficiency can lower costs.
Better Risk Management: It allows for early detection of problems and risks and quick solutions to mitigate any negative impact.
Competitive Advantage: Having a certified QMS can provide a competitive advantage and increase marketing opportunities.
Regulatory Compliance: It ensures compliance with industry-specific standards and regulations.