Digestive System Development
Creative BioMart Digestive System Development Product List
Immunology Background
Background
The digestive system, also referred to as the gastrointestinal (GI) system, is responsible for the digestion and absorption of nutrients and the elimination of waste products. The digestive system comprises the gastrointestinal tract, which includes the mouth, esophagus, stomach, intestines, and anus, as well as accessory organs such as the liver, pancreas, and gallbladder. The development of the digestive system is a complex process that commences in the early stages of embryogenesis and encompasses a multitude of embryonic layers, signaling pathways, and morphological alterations. A comprehensive understanding of the developmental processes governing the digestive system is vital not only for elucidating normal physiological mechanisms but also for the identification and treatment of congenital abnormalities that can have profound clinical implications.
Components of the Digestive System
The digestive system, a complex network of organs and tissues responsible for processing food, extracting and absorbing nutrients, and expelling waste, can be broadly categorized into two main components: the alimentary canal and accessory organs. These structures function in a coordinated manner to ensure that the body receives the nutrients it requires for optimal functioning while simultaneously eliminating indigestible substances.
The Alimentary Canal
The alimentary canal, also known as the gastrointestinal (GI) tract, is a continuous tube that extends from the mouth to the anus. It plays a central role in the digestive process, encompassing several specialized regions, each with distinct functions.
- Mouth: The entry point for food, where mechanical digestion begins with chewing and chemical digestion begins with saliva.
- Esophagus: The esophagus is a muscular tube about 10 inches (25 centimeters) long that connects the pharynx (throat) to the stomach. It transports food by peristalsis and is lined with a protective mucous membrane that shields it from the abrasive effects of passing food.
- Stomach: The stomach is a J-shaped, muscular organ that serves as a temporary holding chamber where food is mixed with gastric juices and then slowly released into the small intestine by the pyloric sphincter.
- Small Intestine: The small intestine is a highly specialized organ, approximately 6 meters in length, where most of the digestion and absorption of nutrients takes place. It is divided into three sections: the duodenum, jejunum, and ileum.
- Large Intestine: The large intestine, which is about 1.5 meters long, plays a crucial role in absorbing water and electrolytes from the remaining indigestible food, and in forming and eliminating feces. It consists of the cecum, colon, and rectum.
- Anus: The anus is responsible for excreting feces and is the terminal end of the digestive tract.
Accessory Organs
In addition to the alimentary canal, the digestive system includes several accessory organs that contribute essential enzymes, hormones, and other substances necessary for digestion. These organs are not part of the GI tract but are crucial for the digestive process.
- Liver: The liver, the largest gland in the body, performs a variety of vital functions, including the production of bile, which is essential for emulsifying and digesting fats. The liver also plays a central role in metabolism, processing nutrients absorbed from the small intestine and detoxifying harmful substances.
- Pancreas: The pancreas is both an exocrine and an endocrine gland. Its exocrine function includes the production of pancreatic juice, which contains digestive enzymes. Its endocrine function includes the secretion of insulin and glucagon, hormones that regulate blood sugar levels.
- Gallbladder: The gallbladder is a small, pear-shaped organ that stores and concentrates bile produced by the liver.
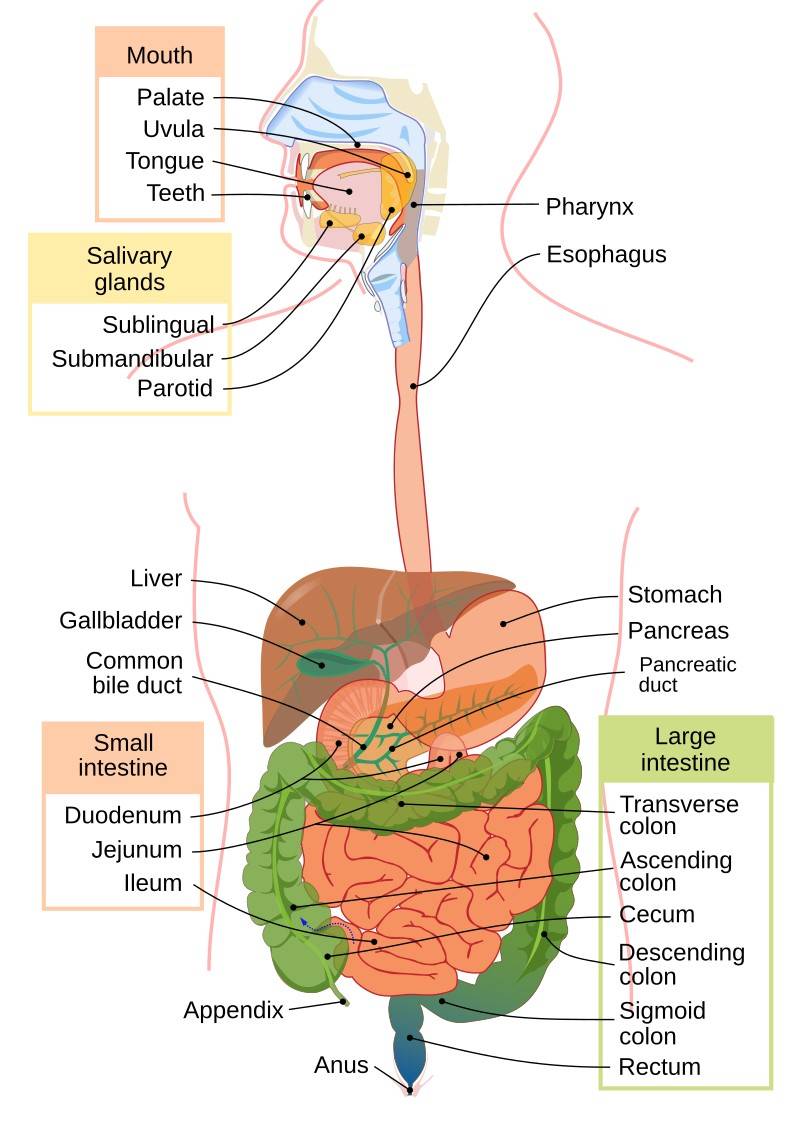 Fig. 1: Human digestive system.
Fig. 1: Human digestive system.Development of the Digestive System
The development of the digestive system begins early in embryogenesis, around the third week of gestation. It is derived primarily from the endoderm, one of the three germ layers formed during gastrulation. The process can be divided into several key stages:
Formation of the Primitive Gut Tube: The primitive gut tube forms from the endoderm during the third week of embryonic development. It is divided into three regions: foregut, midgut, and hindgut, each of which gives rise to different parts of the digestive system.
Differentiation of the Gut Tube: By the fourth week, the gut tube begins to differentiate into the structures that will form the future alimentary canal and accessory organs. The foregut gives rise to the esophagus, stomach, liver, pancreas, and the initial portion of the duodenum. The midgut develops into the small intestine, most of the large intestine, and the appendix. The hindgut forms the distal part of the colon, the rectum, and the anal canal.
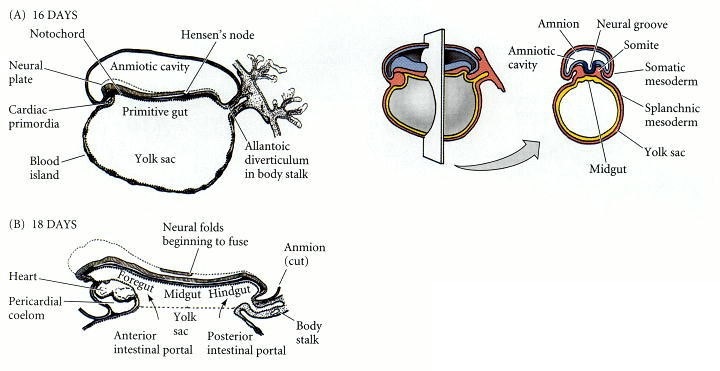 Fig. 2: Formation of the human digestive system, depicted at about (A) 16 days, primitive gut is formed. (B) 18 days, primitive gut divided into foregut, midgut, and hindgut (Developmental Biology, 6th edition).
Fig. 2: Formation of the human digestive system, depicted at about (A) 16 days, primitive gut is formed. (B) 18 days, primitive gut divided into foregut, midgut, and hindgut (Developmental Biology, 6th edition).Vascularization and Innervation: Concurrently, the developing digestive organs are supplied with blood vessels and nerves. The celiac artery, superior mesenteric artery, and inferior mesenteric artery are the main blood supplies to the foregut, midgut, and hindgut, respectively. The enteric nervous system, which is responsible for regulating digestive functions, also begins to form during this period.
Rotation and Fixation: As the intestines grow rapidly, they undergo a series of rotations and fixations within the abdominal cavity. The midgut loop, for example, undergoes a 270-degree counterclockwise rotation that positions the intestines within the abdomen.
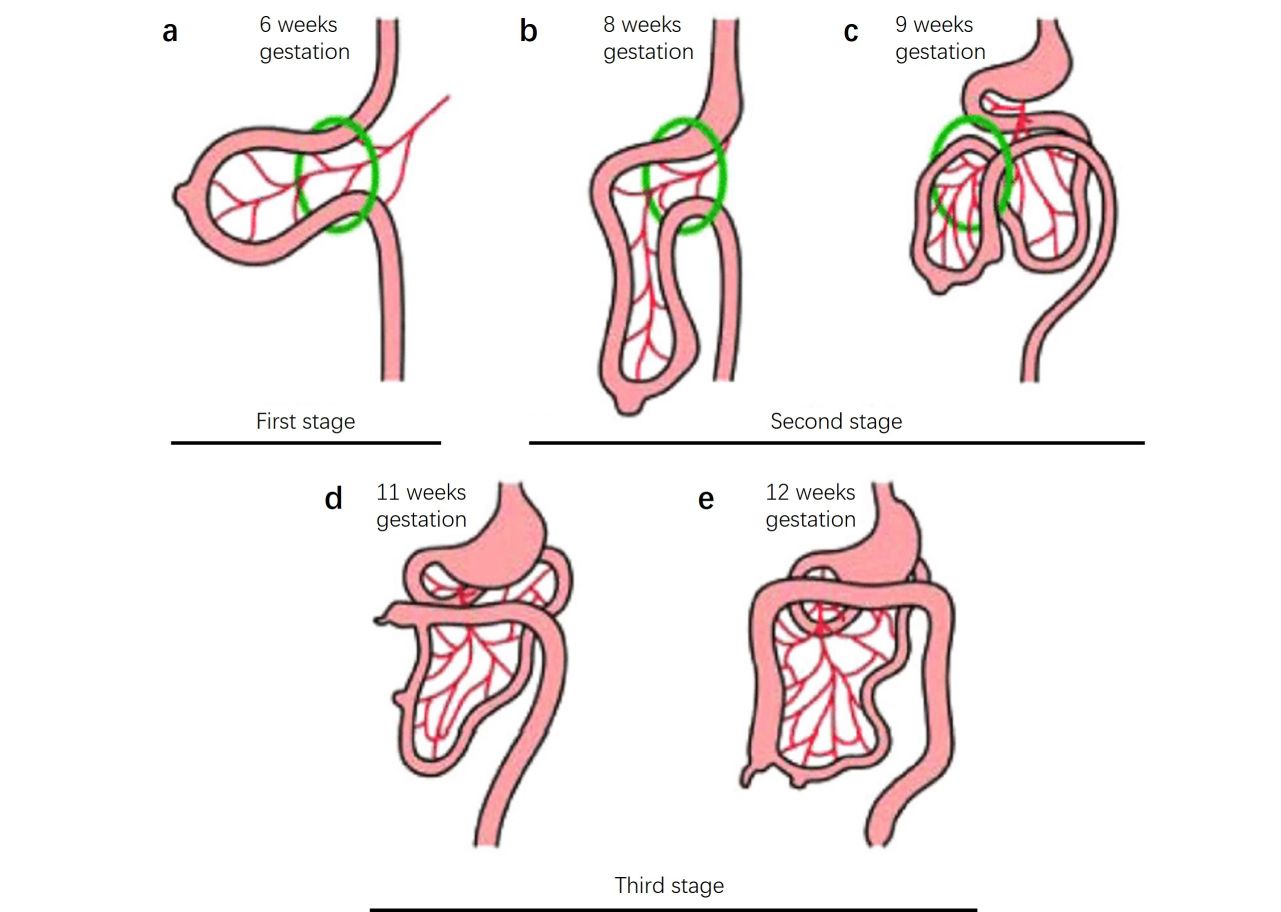 Fig. 3: The three stages of gastrointestinal development. Stage 1 (a), extrusion of the gastrointestinal tract; stage 2 (b, c), beginning of a counterclockwise rotation of the gastrointestinal tract and reentry into the abdomen; stage 3 (d, e), finalization of a total of 270° of counterclockwise rotation of the gastrointestinal tract and final elongation of the colon causing descent and fixation of the cecum (Rittenhouse et al., 2016).
Fig. 3: The three stages of gastrointestinal development. Stage 1 (a), extrusion of the gastrointestinal tract; stage 2 (b, c), beginning of a counterclockwise rotation of the gastrointestinal tract and reentry into the abdomen; stage 3 (d, e), finalization of a total of 270° of counterclockwise rotation of the gastrointestinal tract and final elongation of the colon causing descent and fixation of the cecum (Rittenhouse et al., 2016).Maturation and Functional Development: The digestive system continues to mature and become functional during the later stages of fetal development. By birth, the digestive organs are fully formed and capable of digesting and absorbing nutrients, although certain aspects of digestive function, such as enzyme production, continue to develop after birth.
Regulation of Digestive System Development
The development of the digestive system is a highly orchestrated process, regulated by a complex interplay of genetic, molecular, and cellular signals. These regulatory mechanisms ensure the proper formation, differentiation, and maturation of the various components of the digestive system, from the primitive gut tube to the fully functional gastrointestinal (GI) tract and associated organs.
Genetic Regulation
- Homeobox (Hox) Genes: Hox genes are critical for establishing the anterior-posterior (head-to-tail) axis of the developing embryo. They provide positional information that helps determine which segments of the primitive gut tube will give rise to specific regions of the digestive system, such as the stomach, small intestine, and colon. Different Hox genes are expressed in different patterns along the gut tube and control the development of each region.
- Transcription Factors: A number of transcription factors, such as SOX2, CDX2 and PDX1, play essential roles in specifying the identity of different segments of the intestine. For example, SOX2 is critical for foregut development, while CDX2 is important for midgut and hindgut formation. PDX1 is essential for the development of the pancreas and duodenum.
Molecular Signaling Pathways
- Sonic Hedgehog (SHH) Pathway: The SHH signaling pathway is one of the most important signaling mechanisms in the development of the digestive system. SHH is expressed in the endoderm of the developing gut tube and signals to the surrounding mesoderm, which in turn influences patterning and differentiation of the gut tube. SHH signaling helps regulate the division of the gut into foregut, midgut and hindgut regions and is critical for the proper formation of the stomach, intestine and associated organs.
- Bone Morphogenetic Protein (BMP) Signaling: BMPs are a group of growth factors that play an important role in the development of the GI tract. They are involved in the regulation of cell differentiation, proliferation and apoptosis. BMP signaling is particularly important in foregut development and in patterning the mesoderm surrounding the intestinal tube, which contributes to the formation of the smooth muscle layers of the digestive organs.
- Wnt/β-Catenin Pathway: The Wnt signaling pathway is critical for the proliferation and differentiation of intestinal stem cells, which give rise to the various cell types that line the intestine. Wnt signaling is also involved in the regional specification of the intestinal tube and plays a role in the development of the intestine and colon.
- Fibroblast Growth Factor (FGF) Signaling: FGF signaling is involved in the growth and differentiation of the intestinal tube, particularly in the formation of the liver, pancreas, and lung. FGF signaling gradients help regulate the anterior-posterior patterning of the gut and are important for the development of foregut-derived organs.
Cellular Interactions
- Endoderm-Mesoderm Interactions: The interaction between the endoderm (which forms the lining of the intestinal tube) and the surrounding mesoderm (which forms the connective tissue, smooth muscle, and blood vessels) is crucial for the proper development of the digestive system. Signals from the endoderm, such as SHH, influence the differentiation of the mesoderm, while the mesoderm, in turn, provides signals that promote the differentiation of the endoderm into specific types of epithelial cells.
- Epithelial-Mesenchymal Transition (EMT) and Mesenchymal-Epithelial Transition (MET): These processes are involved in the remodeling of tissues during the development of the digestive system. EMT allows cells to migrate and form new structures, such as the formation of glands and ducts, while MET is important for the reorganization of cells into epithelial layers, such as those lining the gut.
Environmental and Epigenetic Factors
- Epigenetic Regulation: Epigenetic modifications, such as DNA methylation and histone acetylation, play a role in regulating gene expression during the development of the digestive system. These modifications can affect the activity of key developmental genes and are important for the proper temporal and spatial expression of these genes.
- Nutritional and Environmental Influences: The intrauterine environment, including the availability of nutrients and exposure to toxins, can influence the development of the digestive system. Maternal diet and health during pregnancy can have lasting effects on the development and function of the GI tract in the offspring.
Hormonal Regulation
- Growth Hormones and Insulin-Like Growth Factors (IGFs): These hormones play a role in the overall growth and development of the embryo, including the digestive system. In particular, IGFs are involved in the proliferation and differentiation of cells in the intestine.
- Glucocorticoids: These hormones are important in the maturation of the fetal gut, particularly in preparing the digestive system for postnatal function. Glucocorticoids influence the development of enzymes needed for digestion and the maturation of the epithelial lining of the intestine.
Immune System Development
- Gut-Associated Lymphoid Tissue (GALT): The development of the immune system in the gut is critical for protecting the body from ingested pathogens. GALT begins to develop during fetal life and is influenced by interactions between the developing gut and the fetal immune system. This system includes Peyer's patches, isolated lymphoid follicles, and other immune components important to the body's defense mechanisms.
Key Transcription Factors and Cell Markers
Key transcription factors and markers associated with different types of stem and progenitor cells in the digestive system include:
Epithelial Stem Cell Transcription Factors
Epithelial stem cells are responsible for the regeneration and maintenance of epithelial tissues throughout the body, including the gastrointestinal (GI) tract. Important epithelial stem cells transcription factors include:
- SOX9: SOX9 is critical for the maintenance of stem cells in various epithelial tissues, including the intestine. It regulates the self-renewal and differentiation of epithelial stem cells.
- LGR5: LGR5 is a well-known marker of adult stem cells in the intestinal epithelium. It is a target of the Wnt signaling pathway and is essential for the stemness and proliferation of epithelial cells.
- OCT4 (POU5F1): OCT4 is a key transcription factor in maintaining the pluripotency of embryonic stem cells and plays a role in the self-renewal of certain epithelial stem cells.
- BMI1: BMI1 is involved in the regulation of stem cell self-renewal and is associated with epithelial stem cells in various tissues, including the intestine.
Hepatic Progenitor Cell Transcription Factors
Hepatic progenitor cells (HPCs) are bipotent cells capable of differentiating into both hepatocytes and cholangiocytes. Key hepatic progenitor cell transcription factors include:
- HNF4α (Hepatocyte Nuclear Factor 4 Alpha): HNF4α is crucial for liver development and the maintenance of hepatocyte differentiation. It plays a central role in the function of hepatic progenitor cells.
- SOX9: In the liver, SOX9 is expressed in biliary progenitors and is important for cholangiocyte differentiation. It is also involved in the regulation of HPCs.
- PROX1: PROX1 is important for liver development and the regulation of liver-specific gene expression. It is also involved in the differentiation of hepatoblasts into hepatocytes.
- Notch Signaling Pathway: Notch signaling is critical in determining the fate of HPCs, directing them toward cholangiocyte or hepatocyte differentiation.
Intestinal Stem Cell Markers
Intestinal stem cells are essential for the continuous renewal of the intestinal epithelium. Important intestinal stem cell markers include:
- LGR5: LGR5 is the most well-known marker of intestinal stem cells, particularly those located in the crypts of the small intestine and colon. LGR5+ cells are responsible for the continuous renewal of the intestinal mucosa.
- BMI1: BMI1 marks a population of quiescent stem cells in the intestine, which can become active in response to injury.
- OLFM4 (Olfactomedin 4): OLFM4 is a marker of LGR5+ stem cells in the small intestine and colon and is involved in stem cell proliferation and differentiation.
- ASCL2: ASCL2 is a transcription factor that is essential for the identity and maintenance of LGR5+ intestinal stem cells.
Pancreatic Endoderm Cell Markers
Pancreatic endoderm cells are progenitor cells that give rise to the various cell types of the pancreas, including insulin-producing beta cells. Key markers for pancreatic endoderm cells include:
- PDX1 (Pancreatic and Duodenal Homeobox 1): PDX1 is a master regulator of pancreatic development and is essential for the formation of the pancreatic endoderm. It is critical for the differentiation of pancreatic progenitor cells into insulin-producing beta cells.
- NKX6.1: NKX6.1 is another key transcription factor in pancreatic development. It is required for the maturation of beta cells and the maintenance of their function.
- SOX9: SOX9 is important for the development of the pancreatic ductal epithelium and is also expressed in early pancreatic progenitors.
- FOXA2 (Forkhead Box A2): FOXA2 is involved in the regulation of gene expression in the developing pancreas and is important for the differentiation of pancreatic endoderm into mature pancreatic cells.
Pancreatic Progenitor Cell Transcription Factors
Pancreatic progenitor cells are multipotent cells that can give rise to all the cell types of the pancreas, including endocrine, exocrine, and ductal cells. Important pancreatic progenitor cell transcription factors include:
- PDX1: As mentioned earlier, PDX1 is a key regulator of pancreatic progenitor cells, guiding their differentiation into various pancreatic lineages.
- NGN3 (Neurogenin 3): NGN3 is a transcription factor essential for the development of endocrine cells in the pancreas, including insulin-secreting beta cells.
- SOX9: In pancreatic progenitor cells, SOX9 is crucial for maintaining the progenitor state and for ductal cell differentiation.
- HNF6 (Hepatocyte Nuclear Factor 6): HNF6 is important for the development of the pancreatic ducts and for the regulation of NGN3 expression in endocrine progenitors.
Case Study
Case 1: Aigha, I. I.; Abdelalim, E. M. NKX6.1 transcription factor: A crucial regulator of pancreatic β cell development, identity, and proliferation. Stem Cell Research & Therapy, 2020; 11(1), 459.
NKX6.1 is a transcription factor (TF) that plays a critical role in pancreatic β-cell function and proliferation. In human pancreatic islets, NKX6.1 expression is exclusive to β cells and is undetectable in other islet cells. Several reports have shown that activation of NKX6.1 in PSC-derived pancreatic progenitor cells (MPCs) expressing PDX1 (PDX1+/NKX6.1+) guarantees their future commitment to mono-hormonal β cells. However, further differentiation of MPCs lacking NKX6.1 expression (PDX1+/NKX6.1-) results in the undesired generation of non-functional polyhormonal β-cells. This study sheds light on the role of NKX6.1 during pancreatic β cell development and in directing MPCs to the functional mono-hormonal lineage. In addition, we address the transcriptional mechanisms and targets of NKX6.1 and its association with diabetes.
The development of the pancreas and the critical transcription factors during this process are summarized in figure 4 and the function of NKX6.1 during pancreatic development is shown in figure 5.
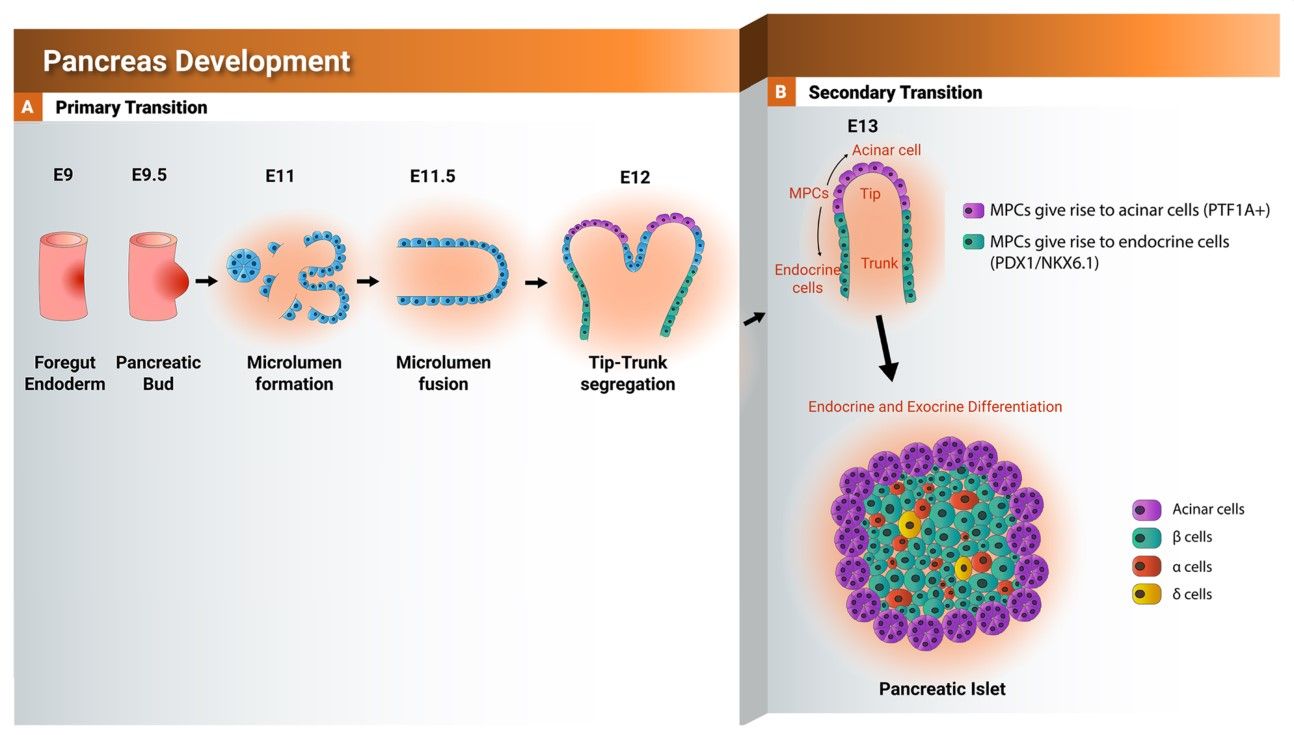 Fig. 4: Schematic representation showing the development of the pancreas from foregut endoderm into pancreatic islet. Tip and trunk domain formation and segregation during the primary transition. During the secondary transition, the formed trunk domain (green) gives rise to the endocrine progenitors and subsequently pancreatic islets, while the tip domain (purple) develops to the exocrine progenitors expressing PTF1A. PTF1A: pancreas associated transcription factor 1a. PDX1: pancreatic and duodenal homeobox 1.
Fig. 4: Schematic representation showing the development of the pancreas from foregut endoderm into pancreatic islet. Tip and trunk domain formation and segregation during the primary transition. During the secondary transition, the formed trunk domain (green) gives rise to the endocrine progenitors and subsequently pancreatic islets, while the tip domain (purple) develops to the exocrine progenitors expressing PTF1A. PTF1A: pancreas associated transcription factor 1a. PDX1: pancreatic and duodenal homeobox 1.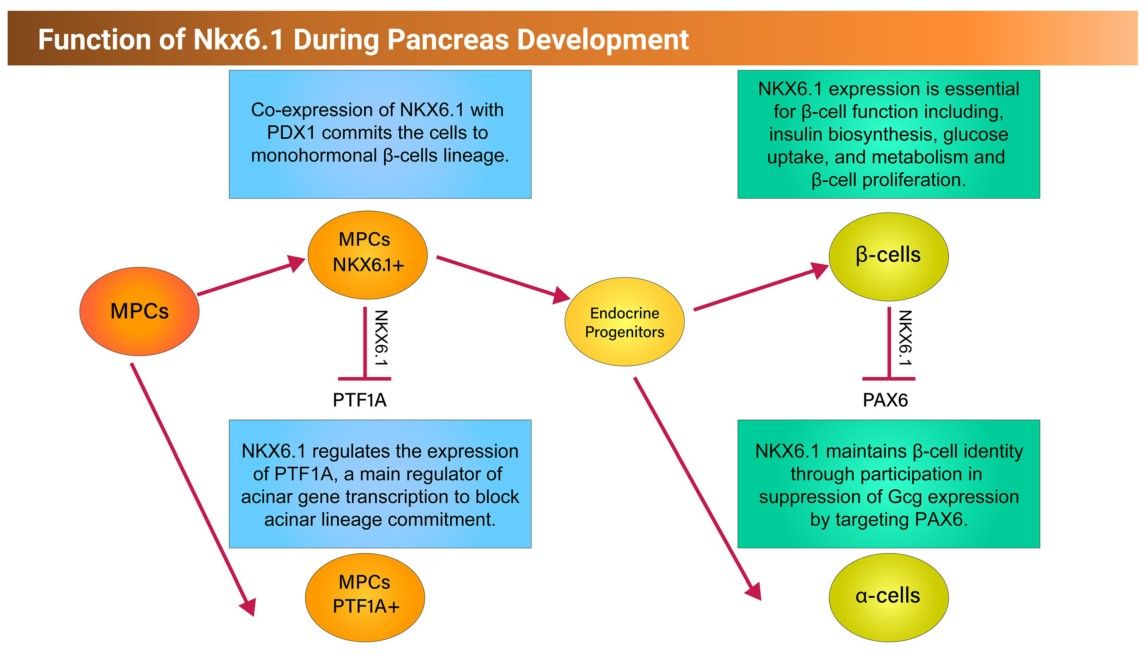 Fig. 5: Schematic representation showing the function of NKX6.1 during early and late pancreatic development.
Fig. 5: Schematic representation showing the function of NKX6.1 during early and late pancreatic development.Case 2: Willet, S. G.; et al. Sox9 governs gastric mucous neck cell identity and is required for injury-induced metaplasia. Cellular and Molecular Gastroenterology and Hepatology, 2023; 16(3), 325–339.
SRY-box transcription factor 9 (Sox9) is a master regulator of digestive system development, it regulates the differentiation of epithelial stem cells. To determine whether Sox9 plays a role in repairing acute and chronic gastric injury, the researchers used immunostaining and electron microscopy to characterize the expression pattern of Sox9 during mouse gastric development, homeostasis, and injury in homeostasis, after genetic deletion of Sox9, and after targeted genetic misexpression of Sox9 in the gastric epithelium and chief cells. Their results showed that Sox9 is critical for mucosal neck cell differentiation during gastric development. Sox9 is also required for chief cells to fully reprogram into spasmolytic polypeptide-expressing metaplasia after injury.
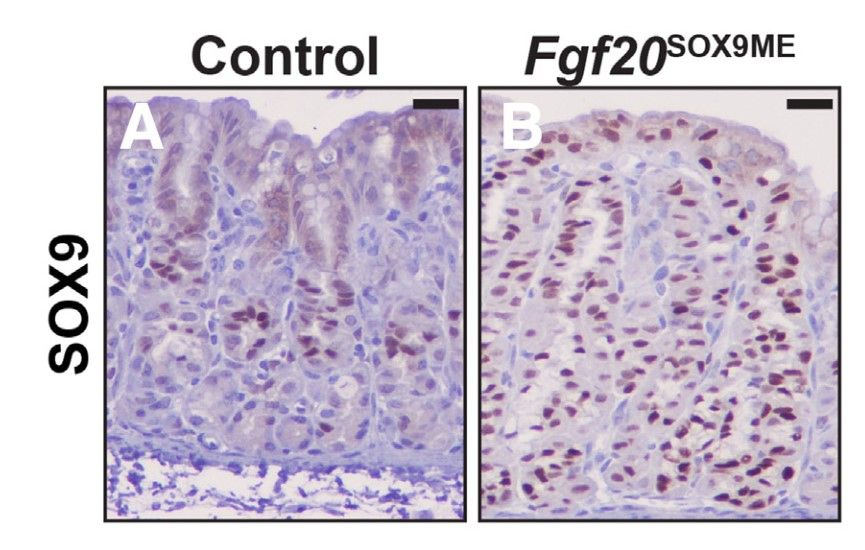 Fig. 6: Postnatal misexpression of Sox9 leads to the expansion of mucous cells. (A) Anti-SOX9 at P21 in control animals shows SOX9 predominately within the isthmus and neck region of corpus units. (B) SOX9 expression in Fgf20SOX9ME animals. Misexpression units express SOX9 from pit to base.
Fig. 6: Postnatal misexpression of Sox9 leads to the expansion of mucous cells. (A) Anti-SOX9 at P21 in control animals shows SOX9 predominately within the isthmus and neck region of corpus units. (B) SOX9 expression in Fgf20SOX9ME animals. Misexpression units express SOX9 from pit to base.References
- Developmental biology (6th ed.) (2000). Sinauer Associates. Gilbert, S. F., & Gilbert, S. F.
- Aigha, I. I., & Abdelalim, E. M. (2020). NKX6.1 transcription factor: A crucial regulator of pancreatic β cell development, identity, and proliferation. Stem Cell Research & Therapy, 11(1), 459.
- Rittenhouse, D. W., Pucci, M. J., Brumbaugh, J. L., Yeo, C. J., & Lavu, H. (2016). Congenital variants of gastrointestinal rotation found at resection of hepatopancreatobiliary tumors: A case series with review of the literature. Case Reports in Pancreatic Cancer, 2(1), 6–13.
- Willet, S. G., Thanintorn, N., McNeill, H., Huh, S.-H., Ornitz, D. M., Huh, W. J., Hoft, S. G., DiPaolo, R. J., & Mills, J. C. (2023). Sox9 governs gastric mucous neck cell identity and is required for injury-induced metaplasia. Cellular and Molecular Gastroenterology and Hepatology, 16(3), 325–339.

