Cardiovascular System Development
Immunology Background
Background
The development of the cardiovascular system represents one of the earliest and most essential stages of embryogenesis, playing a critical role in establishing the foundation for the distribution of oxygen and nutrients to the growing tissues and organs. This complex process commences with the formation of blood vessels through vasculogenesis and the development of the heart, which together establish the initial functional circulatory system in the embryo. The successful formation of the cardiovascular system is essential for the embryo's survival and growth, as it ensures the efficient delivery of essential resources to the developing body.
Vasculogenesis in the Embryo
Vasculogenesis is the de novo formation of blood vessels from mesodermal progenitor cells known as angioblasts. This process begins early in embryonic development, around the third week of gestation in humans. Angioblasts differentiate into endothelial cells, which coalesce to form blood islands. These islands merge to create a primitive vascular network, comprising the first blood vessels.
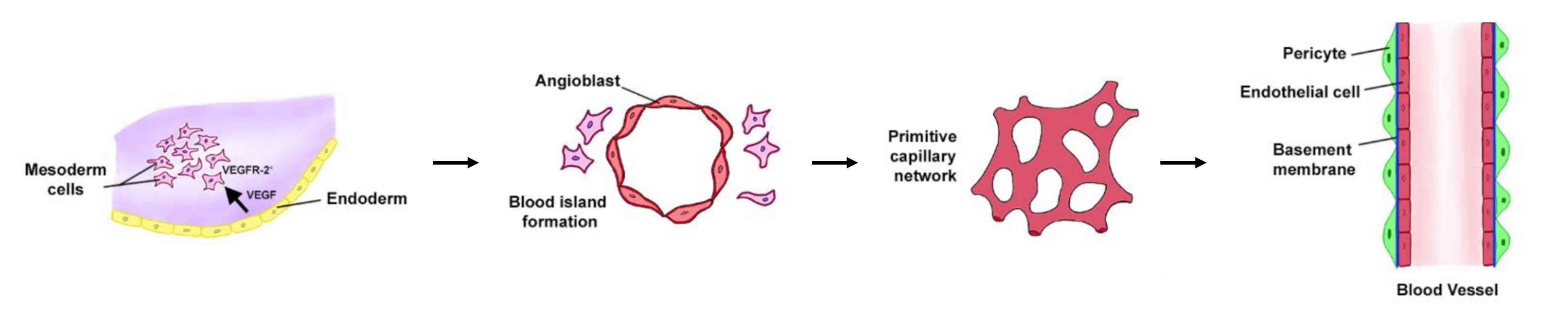 Fig. 1: Vasculogenesis development. Vasculogenesis in embryos is the de novo creation of new vasculature from primitive mesodermal cells that express VEGFR2. VEGF released from neighbouring endodermal cells converts these VEGFR2+ cells into angioblasts surrounding the blood islands. These islands fuse to form the primitive vascular network that eventually converts into the arteriovenous system (Markiewski et al., 2020).
Fig. 1: Vasculogenesis development. Vasculogenesis in embryos is the de novo creation of new vasculature from primitive mesodermal cells that express VEGFR2. VEGF released from neighbouring endodermal cells converts these VEGFR2+ cells into angioblasts surrounding the blood islands. These islands fuse to form the primitive vascular network that eventually converts into the arteriovenous system (Markiewski et al., 2020).Two primary processes occur during this stage: the formation of the dorsal aorta and the development of the cardinal veins. The dorsal aorta forms bilaterally and eventually fuses to form the descending aorta, a critical vessel in the circulatory system. The cardinal veins form the basis of the venous system. These early blood vessels provide the basis for further vascular development and are critical to the survival of the embryo by establishing a circulatory system that supports nutrient and gas exchange.
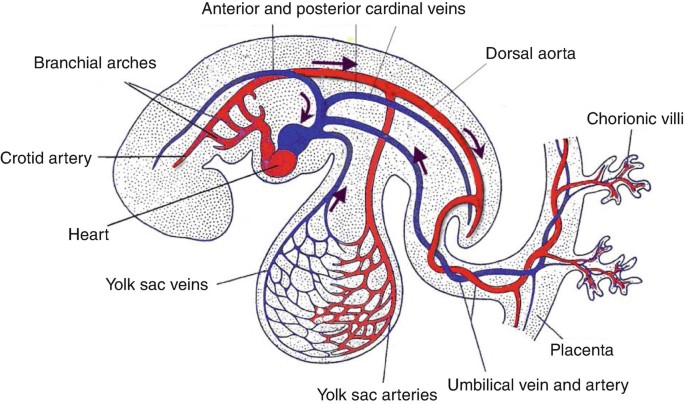 Fig. 2: Illustration of vasculogenesis in the embryo (The Heart and Circulation: An Integrative Model, Springer, 2nd Edition).
Fig. 2: Illustration of vasculogenesis in the embryo (The Heart and Circulation: An Integrative Model, Springer, 2nd Edition).Development of the Heart
The heart is the first functional organ to form during embryogenesis, beginning around the third week of gestation. The development of the heart can be divided into several key stages: cardiac crescent formation, heart tube formation, looping, chamber formation, and septation.
1. Cardiac Crescent Formation: The process starts with the formation of the cardiac crescent, a horseshoe-shaped region of mesodermal cells at the anterior end of the embryo. These cells will give rise to the myocardium, endocardium, and the epicardium.
2. Heart Tube Formation: By the end of the third week, the cardiac crescent cells migrate medially and fuse to form a linear heart tube. This tube consists of an inner endocardial layer, a middle myocardial layer, and an outer epicardial layer.
3. Looping: The heart tube undergoes a process known as looping, where it bends and twists to establish the basic shape of the heart. Looping sets the stage for the alignment of the future chambers and the outflow tract.
4. Chamber Formation: The heart tube then differentiates into the primitive atrium, ventricle, and the bulbus cordis, which will eventually form the right ventricle and the outflow tracts.
5. Septation: The final step in cardiac development is the formation of septation, which divides the heart into four chambers: two atria and two ventricles. This septation is critical for establishing the dual circulatory system required for postnatal life, in which the pulmonary and systemic circuits function independently.
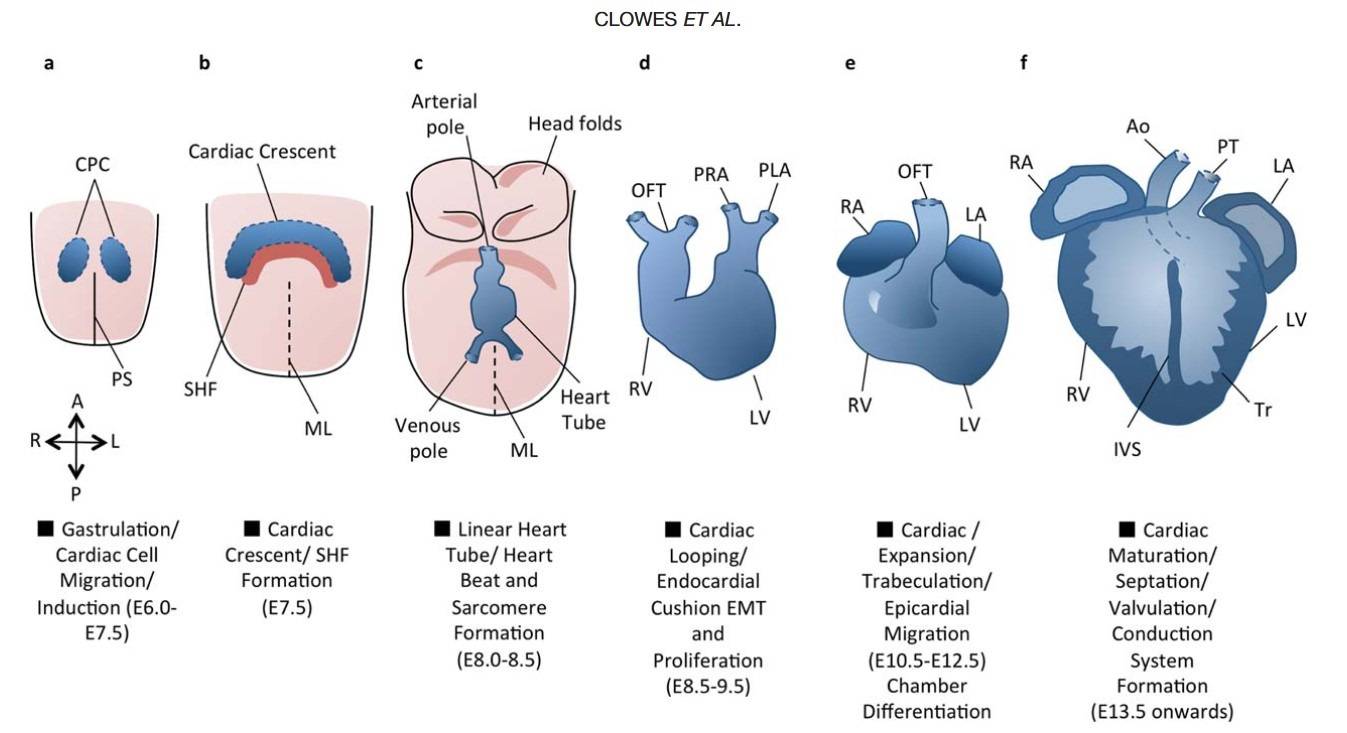 Fig. 3: An overview of cardiac development. Cardiac development progresses from the specification of cardiac progenitor cells (a) to the migration of these cells towards the midline to form the cardiac crescent (b). The developing heart then forms a linear tube (c), which undergoes dextral looping to acquire the appropriate left-right asymmetry (d). The heart tube is further subdivided into the four chambers (e), and the maturation of the endocardial cushions into the valves and development of the great vessels provides for unidirectional blood flow through the chambers (f). A = Anterior, Ao = Aorta, CPC = Cardiac Precursor Cells, IVS = Interventricular septum, L = Left, LA = Left Atrium, LV = Left Ventricle, ML = Midline, OFT = Outflow Tract, P = Posterior, PHF = Primary Heart Field, PLA = Primitive Left Atrium, PRA = Primitive Right Atrium, PS = Primitive Streak, PT = Pulmonary Trunk, R = Right, RA = Right Atrium, RV = Right Ventricle, SHF = Secondary Heart Field, Tr = Trabeculae (Clowes et al., 2014).
Fig. 3: An overview of cardiac development. Cardiac development progresses from the specification of cardiac progenitor cells (a) to the migration of these cells towards the midline to form the cardiac crescent (b). The developing heart then forms a linear tube (c), which undergoes dextral looping to acquire the appropriate left-right asymmetry (d). The heart tube is further subdivided into the four chambers (e), and the maturation of the endocardial cushions into the valves and development of the great vessels provides for unidirectional blood flow through the chambers (f). A = Anterior, Ao = Aorta, CPC = Cardiac Precursor Cells, IVS = Interventricular septum, L = Left, LA = Left Atrium, LV = Left Ventricle, ML = Midline, OFT = Outflow Tract, P = Posterior, PHF = Primary Heart Field, PLA = Primitive Left Atrium, PRA = Primitive Right Atrium, PS = Primitive Streak, PT = Pulmonary Trunk, R = Right, RA = Right Atrium, RV = Right Ventricle, SHF = Secondary Heart Field, Tr = Trabeculae (Clowes et al., 2014).Timecourse of Cardiovascular Development
The timeline of cardiovascular development is highly regulated and sequential, beginning with the formation of blood islands in the third week of gestation. By the fourth week, the primitive heart tube is functional, and blood begins to circulate. The process of heart looping occurs between the fourth and fifth weeks, setting the foundation for the formation of the four-chambered heart.
Septation of the heart chambers begins around the fifth week and continues until the eighth week, by which time the basic structure of the heart is established. The vascular system continues to develop and remodel throughout the remainder of gestation, preparing the fetus for independent circulation after birth.
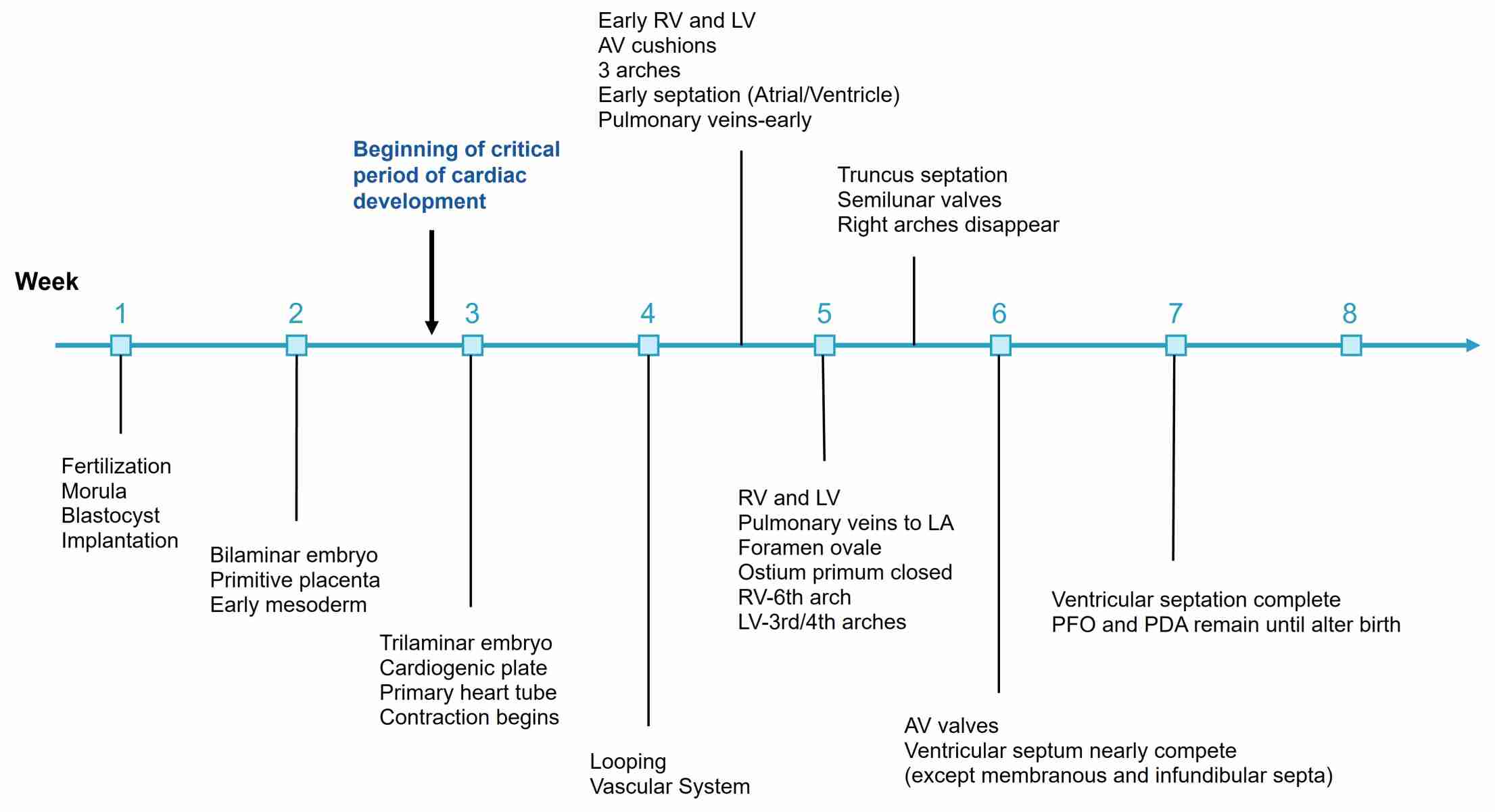 Fig. 4: Timeline of human cardiac development through 8 weeks' gestation.
Fig. 4: Timeline of human cardiac development through 8 weeks' gestation.Pathophysiology and Clinical Significance of Cardiovascular System Development
The development of the cardiovascular system is a complex and highly regulated process, and any disruption or abnormality during this process can lead to significant pathophysiological conditions. Understanding the pathophysiology of cardiovascular development and its clinical significance is critical to the diagnosis, management and prevention of congenital heart disease and other related disorders.
Pathophysiology of Cardiovascular System Development
Congenital Heart Defects (CHDs): One of the most common consequences of abnormal cardiovascular development is congenital heart disease, which occurs in approximately 1% of live births. CHDs result from genetic mutations, environmental factors, or a combination of both that cause malformations of the heart or large blood vessels. Examples include atrial and ventricular septal defects, transposition of the great arteries, tetralogy of Fallot, and hypoplastic left heart syndrome. These conditions can range from mild to life-threatening, depending on the type and extent of the defect.
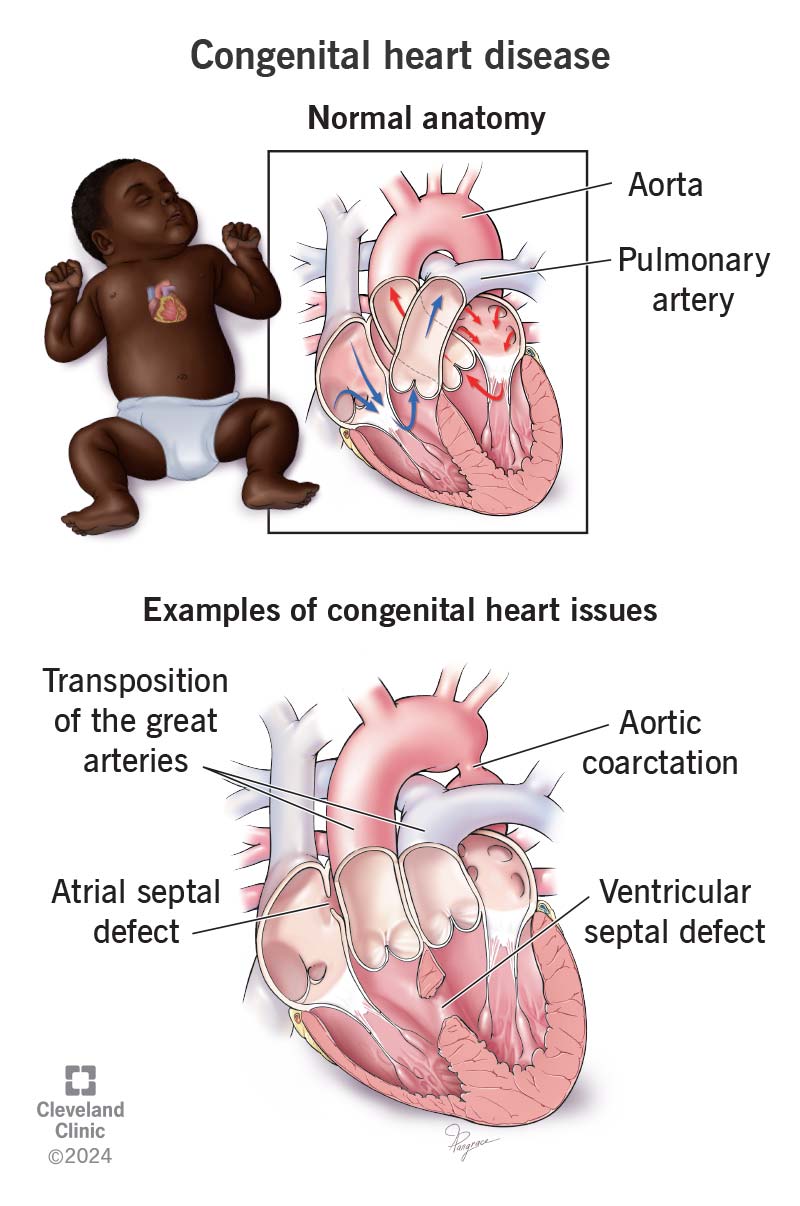 Fig. 5: Examples of congenital heart diseases (Cleveland Clinic).
Fig. 5: Examples of congenital heart diseases (Cleveland Clinic).Disruptions in Vasculogenesis and Angiogenesis: Errors in vasculogenesis (the formation of initial blood vessels) and angiogenesis (the growth of new blood vessels from existing ones) can result in inadequate blood supply to the developing embryo. This can lead to ischemia and tissue hypoxia, resulting in developmental delays, organ dysfunction, or even embryonic lethality in severe cases.
Genetic and Epigenetic Factors: Genetic mutations affecting key regulatory genes such as NKX2.5, GATA4, and TBX5 can disrupt normal heart development. Epigenetic modifications, including DNA methylation and histone modification, also play a significant role in the regulation of gene expression during heart development. Abnormalities in these processes can lead to structural defects and functional impairments in the cardiovascular system.
Hemodynamic Disturbances: Abnormal blood flow patterns during fetal development can lead to structural abnormalities of the heart and blood vessels. For example, improper closure of the foramen ovale or the ductus arteriosus can result in conditions such as patent foramen ovale (PFO) or patent ductus arteriosus (PDA), which may cause complications later in life.
Environmental Influences: Maternal factors such as diabetes, obesity, infections, and exposure to teratogens (e.g., alcohol, certain medications) during pregnancy can interfere with normal cardiovascular development. These factors can lead to an increased risk of CHDs and other cardiovascular abnormalities.
Clinical Significance of Cardiovascular System Development
Early Detection and Diagnosis: Understanding the pathophysiology of cardiovascular system development has led to advancements in prenatal screening and diagnosis. Techniques such as fetal echocardiography and genetic testing allow for the early detection of CHDs and other cardiovascular abnormalities, enabling timely intervention and better outcomes.
Management of Congenital Heart Defects: Knowledge of cardiovascular development is essential for the surgical and medical management of CHDs. Surgical interventions, such as septal defect repair or arterial switch surgery, are often required to correct structural defects. Pharmacological treatments, including diuretics, beta-blockers, and prostaglandin inhibitors, are used to manage symptoms and prevent complications.
Prevention Strategies: Understanding the risk factors associated with abnormal cardiovascular development has led to preventive measures. For example, folic acid supplementation during pregnancy has been shown to reduce the risk of certain congenital heart defects. Additionally, managing maternal health conditions such as diabetes and avoiding teratogenic substances can help prevent CHDs.
Long-Term Outcomes and Follow-Up: Individuals born with CHDs may require lifelong follow-up care to monitor for potential complications, such as arrhythmias, heart failure, or pulmonary hypertension. Knowledge of cardiovascular development helps clinicians anticipate these issues and provide appropriate care throughout the patient's life.
Research and Therapeutic Advances: Ongoing research into the molecular mechanisms of cardiovascular development is leading to the discovery of new therapeutic targets and regenerative medicine approaches. For example, stem cell therapy and tissue engineering hold promise for repairing or replacing damaged heart tissue, offering hope for individuals with severe congenital or acquired heart disease.
Case Study
Case 1: Galvin, K. M.; et al. A role for Smad6 in development and homeostasis of the cardiovascular system. Nature Genetics, 2000; 24(2), 171-174.
Smad proteins are intracellular mediators of signaling initiated by Tgf-β superfamily ligands (Tgf-βs, activins and bone morphogenetic proteins (Bmps)). Smads are activated upon phosphorylation by specific type I receptors, and associate with the common partner Smad to trigger transcriptional responses. In this study, the researchers explored the role of an inhibitory Smad in vivo by targeted mutation of Madh6 (which encodes the Smad6 protein).
Targeted insertion of a LacZ reporter revealed that Smad6 expression is largely restricted to the heart and blood vessels and that Madh6 mutants exhibit multiple cardiovascular abnormalities. Heart valve hyperplasia and outflow tract septation defects suggest a function for Smad6 in regulating endocardial cushion transformation. The role of Smad6 in the homeostasis of the adult cardiovascular system is indicated by the development of aortic ossification and elevated blood pressure in viable mutants. These defects highlight the importance of Smad6 in the tissue-specific modulation of Tgf-β superfamily signaling pathways in vivo.
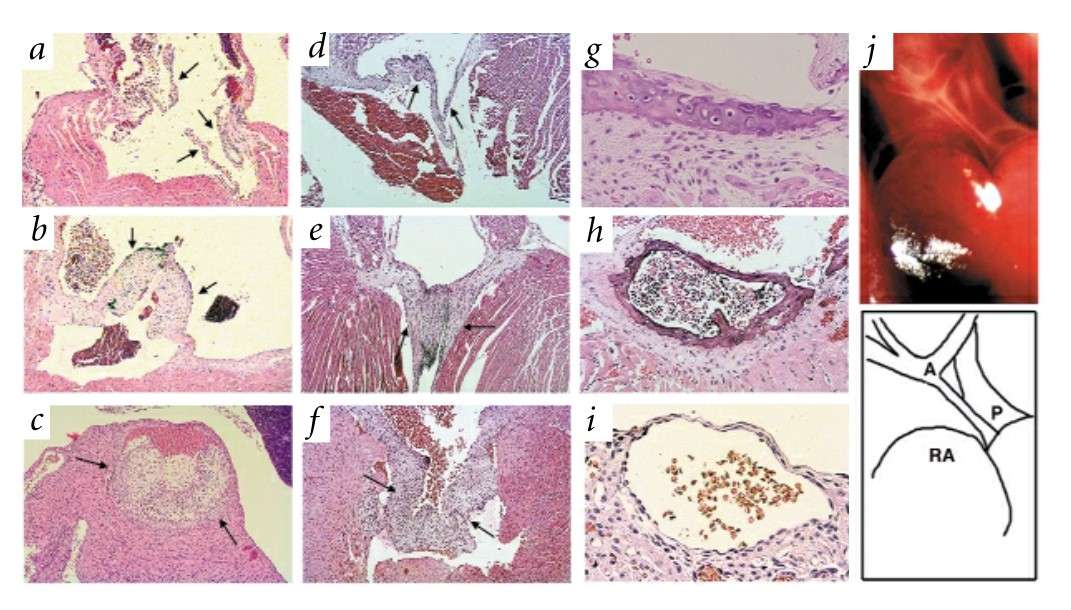 Fig. 6: Pathology of Madh6-mutant mice. The Madh6-/- mice shown are progeny of both heterozygous and homozygous mutant parents on a mixed 129/SvEv x BALB/cBy background. a-f, Transverse sections through cardiac valves. Arrows indicate valve leaflets. Wild-type adult (a) and mutant adult (b) pulmonary valves are shown. c, Extreme hyperplastic pulmonary valve from a moribund eight-day-old mouse. Wild-type adult (d), mutant adult (e) and 14-day-old mutant (f) mitral valves are shown. g, Cartilaginous metaplasia of the aortic media. h, Advanced medial ossification with marrow. i, Subepicardial vascular malformation in the right ventricular wall of a dead newborn. j, Abnormal outflow tract (schematic below; A indicates aortic arch, note narrow ascending aorta; P indicates enlarged pulmonary trunk; RA indicates right atrium) in a dead newborn.
Fig. 6: Pathology of Madh6-mutant mice. The Madh6-/- mice shown are progeny of both heterozygous and homozygous mutant parents on a mixed 129/SvEv x BALB/cBy background. a-f, Transverse sections through cardiac valves. Arrows indicate valve leaflets. Wild-type adult (a) and mutant adult (b) pulmonary valves are shown. c, Extreme hyperplastic pulmonary valve from a moribund eight-day-old mouse. Wild-type adult (d), mutant adult (e) and 14-day-old mutant (f) mitral valves are shown. g, Cartilaginous metaplasia of the aortic media. h, Advanced medial ossification with marrow. i, Subepicardial vascular malformation in the right ventricular wall of a dead newborn. j, Abnormal outflow tract (schematic below; A indicates aortic arch, note narrow ascending aorta; P indicates enlarged pulmonary trunk; RA indicates right atrium) in a dead newborn.Case 2: Groenendijk, B. C. W.; et al. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Developmental Dynamics, 2004; 230(1), 57-68.
Blood flow patterns significantly influence cardiovascular development, and changes can lead to congenital heart defects. Shear stress, which correlates with blood flow, is thought to impact cardiac development. This study examined the expression patterns of ET-1, NOS-3, and KLF-2 in developing chicken embryos, as these genes respond to shear stress. KLF-2, associated with high shear stress, was found to overlap with NOS-3 expression in narrow cardiovascular regions, while ET-1 expression was low or absent in these areas. These results suggest that KLF-2, NOS-3, and ET-1 are involved in shear stress responses during cardiovascular development, with implications for understanding congenital heart conditions.
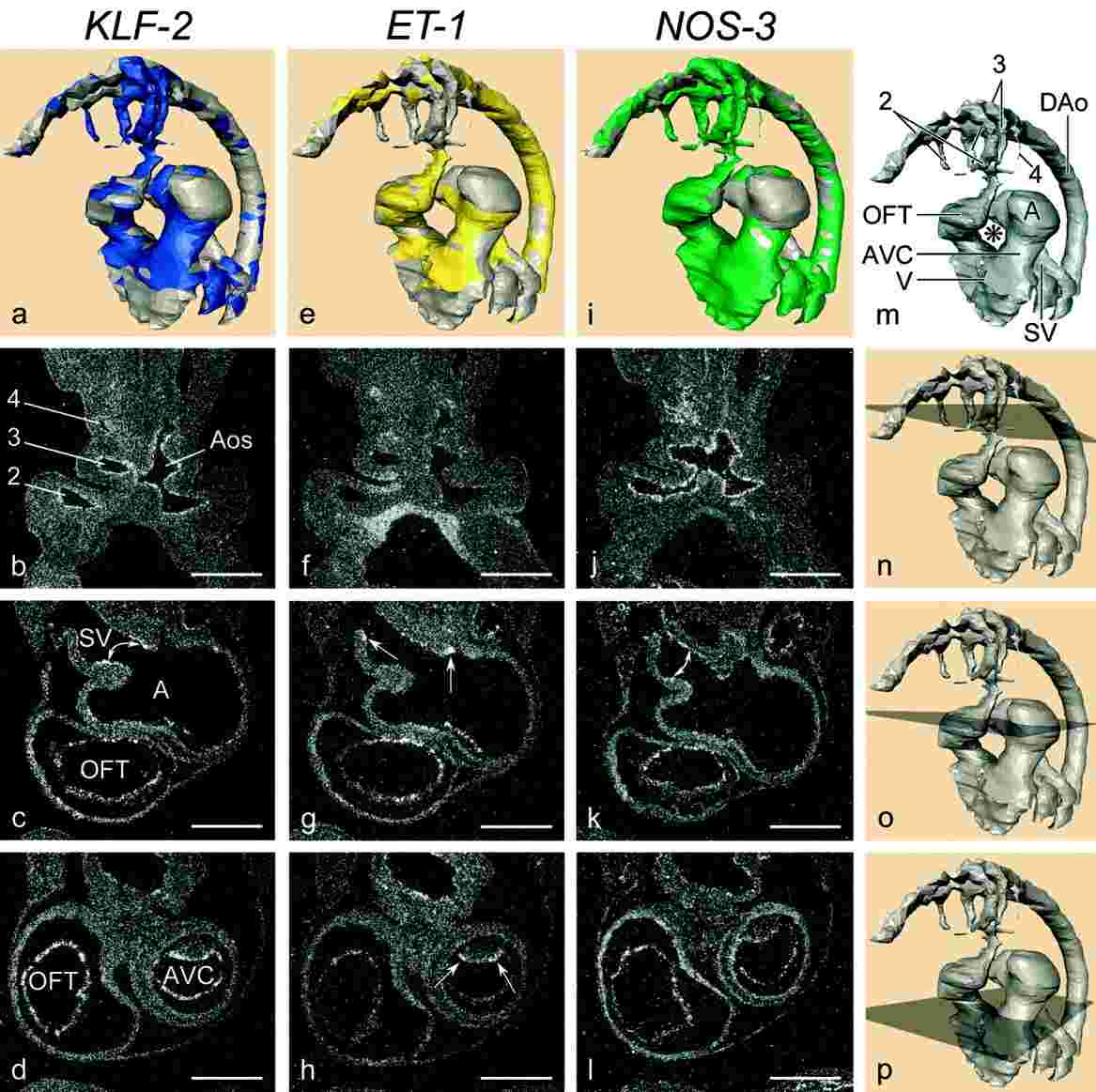 Fig. 7: a-p: Localisation of KLF-2, ET-1, and NOS-3 at Hamburger and Hamilton stage 18. Note the presence of KLF-2 and NOS-3 and the absence of ET-1 in the sinus horns (compare a, e, i). Also note the positive ET-1 and negative KLF-2 and NOS-3 in the SV. c, g, and k demonstrate KLF-2 and NOS-3 expression in the narrow part of the sinoatrial transition, ET-1 is detected both upstream and downstream (arrows). In the AVC (d, h, l) along the inner curvature (as compiled in a, e, i), all three messages are present. h: ET-1 shows expression at the lateral side of the cushion (arrows), the flat top shows weaker expression. i: NOS-3 is detected along the ventricular trabeculations, unlike the other two genes (compare a, e). The proximal OFT shows excluding expression of KLF-2 with ET-1 (a, e), NOS-3 is weakly expressed. In the distal OFT, messages overlap with each other (compilation in a, e, i). In the Aos and pharyngeal arch arteries, KLF-2 (a, b) andNOS-3 (i, j) are present, in contrast to ET-1 (e,f). ET-1 and NOS-3 are excluding with KLF-2 in the DAo (compare a, e, i). The asterisk in m indicates the inner curvature. 2, second pharyngeal arch artery; A, atrium; Aos, aortic sac; AVC, atrioventricular canal; DAo, dorsal aorta; OFT, outflow tract; SV, sinus venosus; V, ventricle. Scale bars = 200 μm in b- d, in f- h, in j-l.
Fig. 7: a-p: Localisation of KLF-2, ET-1, and NOS-3 at Hamburger and Hamilton stage 18. Note the presence of KLF-2 and NOS-3 and the absence of ET-1 in the sinus horns (compare a, e, i). Also note the positive ET-1 and negative KLF-2 and NOS-3 in the SV. c, g, and k demonstrate KLF-2 and NOS-3 expression in the narrow part of the sinoatrial transition, ET-1 is detected both upstream and downstream (arrows). In the AVC (d, h, l) along the inner curvature (as compiled in a, e, i), all three messages are present. h: ET-1 shows expression at the lateral side of the cushion (arrows), the flat top shows weaker expression. i: NOS-3 is detected along the ventricular trabeculations, unlike the other two genes (compare a, e). The proximal OFT shows excluding expression of KLF-2 with ET-1 (a, e), NOS-3 is weakly expressed. In the distal OFT, messages overlap with each other (compilation in a, e, i). In the Aos and pharyngeal arch arteries, KLF-2 (a, b) andNOS-3 (i, j) are present, in contrast to ET-1 (e,f). ET-1 and NOS-3 are excluding with KLF-2 in the DAo (compare a, e, i). The asterisk in m indicates the inner curvature. 2, second pharyngeal arch artery; A, atrium; Aos, aortic sac; AVC, atrioventricular canal; DAo, dorsal aorta; OFT, outflow tract; SV, sinus venosus; V, ventricle. Scale bars = 200 μm in b- d, in f- h, in j-l.References
- The Heart and Circulation: An Integrative Model, Springer, 2nd Edition, 2014. Furst, B.
- Galvin, K. M., Donovan, M. J., Lynch, C. A., Meyer, R. I., Paul, R. J., Lorenz, J. N., Fairchild-Huntress, V., Dixon, K. L., Dunmore, J. H., Gimbrone, M. A., Falb, D., & Huszar, D. (2000). A role for Smad6 in development and homeostasis of the cardiovascular system. Nature Genetics, 24(2), 171-174.
- Groenendijk, B. C. W., Hierck, B. P., Gittenberger-de Groot, A. C., & Poelmann, R. E. (2004). Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Developmental Dynamics, 230(1), 57-68. https://doi.org/10.1002/dvdy.20029
- Clowes, C., Boylan, M. G. S., Ridge, L. A., Barnes, E., Wright, J. A., & Hentges, K. E. (2014). The functional diversity of essential genes required for mammalian cardiac development. Genesis, 52(8), 713-737.
- Markiewski, M. M., Daugherity, E., Reese, B., & Karbowniczek, M. (2020). The role of complement in angiogenesis. Antibodies, 9(4), 67.

