PEGylation Services
Background
What is PEGylation?
Polyethylene glycol is a safe, immune-neutral polymer that dissolves easily in water. Its structure features repeating units of ethylene glycol, providing it with unique flexibility and protective qualities. When applied through PEGylation, PEG chains can mask a molecule, reducing recognition and removal by the immune system and thus decreasing immunogenicity, which extends its presence in the bloodstream. These chains also boost the molecule’s water solubility and stability by increasing its hydration layer, ensuring resilience in various physiological conditions.
PEGylation operates through chemical coupling reactions or physical adsorption. This involves reactive PEG derivatives—like those with carboxyl, amino, or maleimide groups—covalently attaching to specific locations on target molecules, such as the amino or thiol groups of proteins. This targeted modification preserves the molecule’s functional integrity while enhancing its pharmacokinetic profile. For instance, PEGylated protein drugs generally show longer plasma half-lives, reduced toxicity, and greater therapeutic effectiveness.
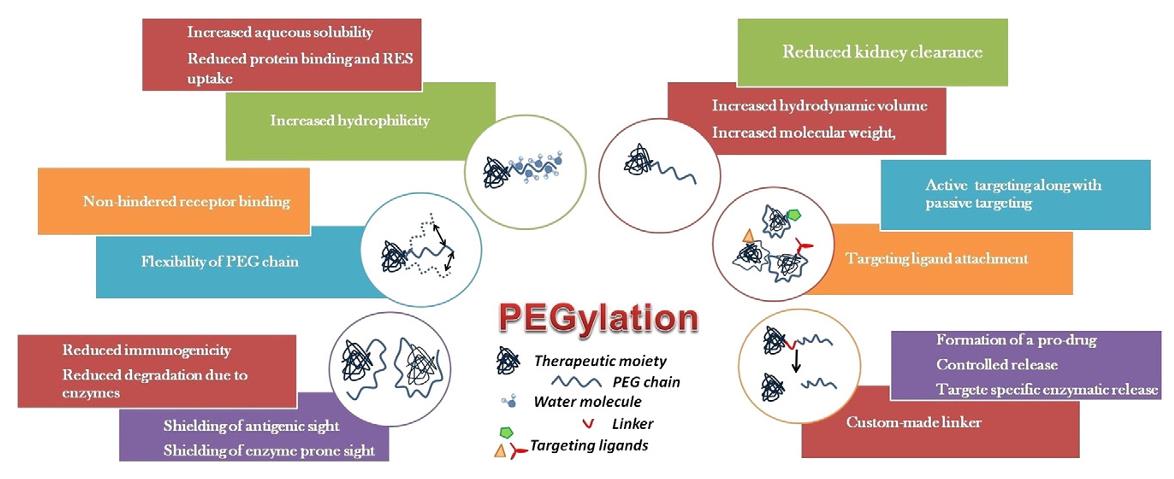
Fig. 1. Outcomes of PEGylation. (Kolate, et al., 2014)
What is PEGylation Used for?
PEG modification is one of the major technologies applied to protein applications, particularly in pharmacokinetic and pharmacodynamic analysis of therapeutic proteins. The technique increases the activity of the protein by making it more lubricous, stable, and extend its half-life while also decreasing its immunogenicity and proteolysis.
Protein Therapy : Pegylation is common to a variety of proteins – interferon, growth hormones, enzymes – in order to optimise their effectiveness. PEG-intron® (PEGylated interferon alpha-2B) and Pegasys® (PEGylated interferon alpha-2A), for instance, show longer cycle times and lower dose requirements, because pegylated interferon alpha-2A chains are used. Furthermore, pegylated asparaginases like Oncaspar® are also useful in acute lymphoblastic leukemia.
Site-Specific PEGylation : Site-specific PEGylation now enables PEG chains to be attached to a location in the protein such as amino or thiol, by chemical conjugation. This accuracy reduces PEGylated product heterogeneity and increases therapy efficacy. For instance, thiol-selective conjugation with cysteine residues is gaining attention for its specificity and ability to maintain protein function.
Protein Stability and Delivery : PEGylation protects proteins from aggregation and denaturation, ensuring stability during storage and administration. This has applications in protein drug delivery, where PEGylated proteins show improved absorption and controlled release properties.
Lower Immunogenicity: PEGylation obfuscates antigenic areas on proteins, greatly reducing the immune response. That is why it’s an ideal agent for long-term treatments, such as PEGylated granulocyte colony-stimulating factors (Neulasta®).
Diagnostics and Imaging : PEGylated proteins are also used in diagnostics, where their modified properties enhance detection sensitivity in techniques such as ELISA and in vivo imaging. PEGylated fluorescent dyes improve solubility and clearance, facilitating precise cellular and tissue-level imaging.
Online InquiryService Procedure
-

FT-IR Spectrometer
-

Large-scale Bioreactors
-

Mass Spectrometer
Service Details
Our PEGylation Feasibility Assessment Service provides a comprehensive molecular assessment to determine the best PEG strategy and potential benefits for a drug or biomolecule supplied by our client. Through a combination of molecular modeling and biosimulation, we provide data-driven modification recommendations to ensure that the PEG process accurately meets the molecular characteristics. In addition, we will provide detailed technical feasibility reports and cost projections to help customers make informed decisions prior to PEgging.
Our Pilot Production and Scale-Up Support Service is designed to guide clients through the transition from laboratory to pilot-scale production of PEGylated drugs. We offer GMP-compliant small-batch trial production, ensuring that all processes meet stringent industry regulatory requirements. This service is not just about production; it includes comprehensive process scale-up validation, covering optimization of production parameters and analysis of product consistency. Our expertise lies in supporting the trial production of complex molecular systems, ensuring that the scalability of PEGylation processes is achieved with precision and reliability. By focusing on the intricacies of complex molecules, we help clients navigate the challenges of scaling up production while maintaining the quality and efficacy of their PEGylated therapeutics.
Our PEGylation Service uses cutting-edge tech to help you find the best PEGylation strategies and figure out what’s in your conjugates. We can confirm molecular structures with tools like HPLC, LC-MS, and NMR, and we also test how well the PEGylated products work to make sure they’re effective and reliable. Our service stands out with customized screening experiments that optimize the modification sites and the length of PEG chains, tailoring the PEGylation process to the specific needs of each molecule. The comprehensive report we provide includes analysis of modification homogeneity, purity, and stability, giving clients a clear understanding of the characteristics of their PEGylated conjugates and the confidence to move forward with development and production.
Our PEGylated Biosimilar Investigation Service covers everything you need for PEGylating biosimilars, from initial ideas to final product tweaks. We’re pros at fine-tuning PEGylation methods to make biosimilars less likely to cause immune reactions and work even better. We also give you a leg up with market analysis and side-by-side checks of similar products, helping you make smart choices. Plus, we’ve got your back with research and preclinical support to set the stage for developing effective PEGylated biosimilars.
Our Analytical Method Development and Validation Service is all about designing and pre-testing really accurate ways to check PEGylated products. We focus on crafting scalable methods to spot impurities and ensure consistency and purity during production. Plus, we handle bioactivity and stability testing to see how well the products perform and how long they last. We’re dedicated to meeting the strict standards set by agencies like the FDA and EMA, so our clients can trust their PEGylated products meet global regulations.
Our Custom PEGylation Process Design Service focuses on developing personalized PEGylation methods to suit the unique characteristics of your target molecules and your specific needs. We tailor everything from site specific modification techniques to fine tune reaction conditions to get the best results. Whether you're looking for linear or multi-arm PEGylation, we've got your full range of molecular requirements. In addition, our flexible process development ensures that everything from design to production is seamless, so there are no obstacles to lift and integration into the manufacturing process.
Online InquiryOur Advantages
- Modular service design : Flexible combination to meet customer specific project needs.
- Full process coverage : from early research to trial production, analysis and verification, to support customer products to accelerate the market.
- Advanced technology : The introduction of the latest site-specific modifications and high-throughput analysis methods.
- GMP compatibility : Provide standardized production support from pilot production to commercialization.
- Personalized support : Combined with industry trends, with a particular focus on the PEGylation needs of biosimilars and emerging areas such as nucleic acid drugs.
Case Study
Case Study 1: 1 H NMR spectroscopy for quantifying PEGylated biomolecules in biological fluids.
When it comes to how well therapeutic agents work, things like how they accumulate, spread in the body, and get cleared out are crucial. Figuring this out is a big experiment challenge. Many treatments, from small molecules to proteins and drug carriers, are often linked with PEG to boost their performance and stability in the body. Here researchers found that 1 H NMR spectroscopy is a game-changer for measuring PEGylated compounds in complex body fluids quickly and with little prep work. PEG stands out with its unique proton signal, letting detect even tiny amounts—like 10 μg/mL in blood. Using special techniques like 13 C-enriched PEG and advanced filtering, researchers can detect even lower levels. They shown this works in animal blood, and can track how PEG and PEGylated substances clear out of a live rat’s system. This method works for various PEGylated biomolecules, making it easier to measure their distribution and clearance without complicated steps.
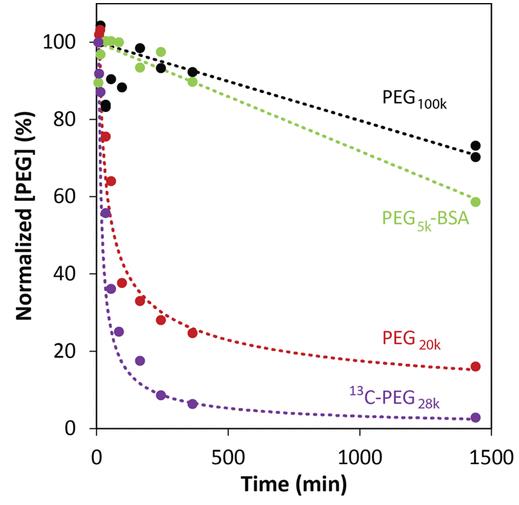
Fig. 2. Rat blood clearance profiles for various PEG species as monitored via 1 H NMR. (Alvares, et al., 2016)
Case Study 2: Innovative approach to enhance PEGylated protein bioactivity.
PEGylating protein ligands, like adding polyethylene glycol (PEG) to therapeutic proteins, can boost their half-life but often reduces bioactivity. This bioinspired strategy tackles this issue by attaching a PEG 30kDa -modified protease-sensitive peptide linker (PSL) to a specific part of insulin-like growth factor I (IGF-I). This strategy uses both enzymatic and chemical coupling methods. The PSL can be specially targeted and cleaved in diseased tissues by matrix metalloproteinases (MMP), effectively restoring the IGF-I’s original properties in those areas. This IGF-PSL-PEG conjugate shows different binding affinities and behaviors compared to its natural form, but can revert to its wild type functions when exposed to the elevated MMP levels found in inflamed tissues.
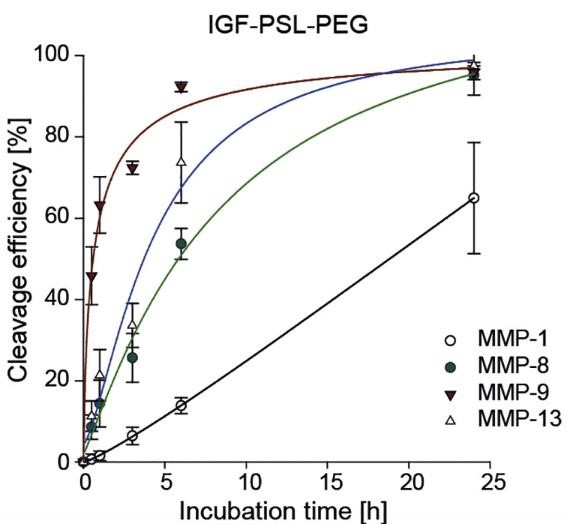
Fig. 3. Time-dependent cleavage of IGF-PSL-PEG exposed to MMP-1, -8, -9 and -13 and analyzed by RP-HPLC. (Braun, et al., 2018)
Case Study 3: Effects of PEGylation on antimicrobial peptide LyeTx I-b cys .
PEGylation, the attachment of polyethylene glycol (PEG) chains to molecules, has grown in popularity for reducing toxicity in protein and peptide drugs, but it may affect their structure and biological activity. The antimicrobial peptide LyeTx I-b cys was synthesized and PEGylated with a 2.0 kDa PEG. This modification reduced cytotoxicity in VERO cells and altered its binding affinity to anionic membranes. Microbiological tests showed that the PEGylated version (LyeTx I-bPEG) was less effective against bacteria like Staphylococcus aureus and Escherichia coli compared to non-PEGylated LyeTx I-b cys . Despite these differences, PEGylation did not significantly change the peptide’s overall structure, indicating minimal impact on its conformational freedom.
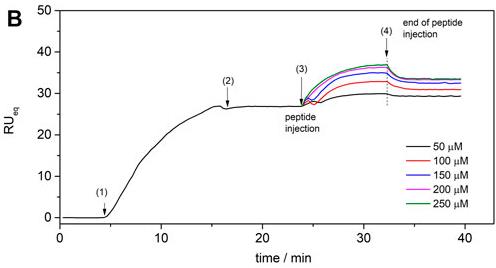
Fig. 4. SPR sensograms for the bilayer interaction of LyeTx I-bPEG. (Moreira Brito, et al., 2022)
Other Resources
FAQs
-
Q: Can I pick a specific kind of PEG?
A: Absolutely, we’ve got all sorts of PEG types like linear, branching, dual-function, etc., and different molecular weights ranging from 5kDa up to 40kDa. We’ll help you find the perfect one for what you need.
-
Q: Will PEGylation mess with the protein’s activity?
A: We use site-specific modification tech to keep from fiddling with crucial active sites. That way, the PEGylated protein keeps doing its biological job just fine.
-
Q: Can I test the effects of different PEG-modification schemes?
A: We provide PEG-strategy screening services to test the effect of different modification schemes on target molecules, including modification position, chain length and chemical groups.mical groups.
-
Q: My product will be used in clinical trials. Does PEGylation meet regulatory requirements?
A: Our analytical and manufacturing services comply with ICH guidelines and relevant regulatory requirements, and are supported by a complete technical package that meets FDA or EMA standards.
References:
- Kolate A.; et al . PEG - a versatile conjugating ligand for drugs and drug delivery systems. J Control Release . 2014;192:67-81.
- Alvares RD.; et al . Quantitative detection of PEGylated biomacromolecules in biological fluids by NMR. Anal Chem . 2016;88(7):3730-3738.
- Braun AC.; et al. Bioresponsive release of insulin-like growth factor-I from its PEGylated conjugate. J Control Release . 2018;279:17-28.
- Moreira Brito JC.; et al . PEGylation of the antimicrobial peptide LyeTx I-b maintains structure-related biological properties and improves selectivity. Front Mol Biosci . 2022;9:1001508.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

