OSM
-
Official Full Name
oncostatin M -
Overview
Oncostatin M, also known as OSM, is a protein that in humans is encoded by the OSM gene. OSM is a pleiotropic cytokine that belongs to the interleukin 6 group of cytokines. Of these cytokines it most closely resembles leukemia inhibitory factor (LIF) in both structure and function. However, it is as yet poorly defined and is proving important in liver development, haematopoeisis, inflammation and possibly CNS development. It is also associated with bone formation and destruction. -
Synonyms
OSM;oncostatin M;MGC20461;oncostatin-M;OTTHUMP00000198700
Recombinant Proteins
- Human
- Bovine
- Canine
- Mouse
- Swine
- Rhesus macaque
- Rat
- Rhesus Monkey
- E.coli
- Yeast
- HEK293
- Human Cells
- Mammalian Cells
- Human
- Insect Cells
- His
- Non
- Fc
- Strep II
- GST
- Avi
- DDK
- Myc
- Flag
Background
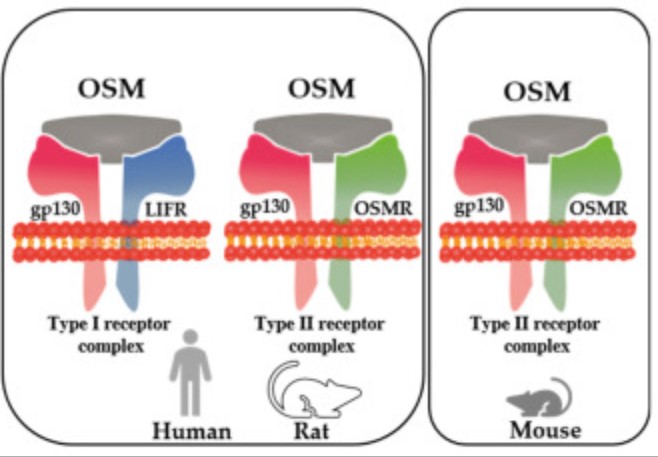
Fig1. The differential formation of receptor complexes by OSM in the human, rat, and mouse. (Thomas Kubin, 2022)
What is OSM protein?
OSM (oncostatin M) gene is a protein coding gene which situated on the long arm of chromosome 22 at locus 22q12. This gene encodes a member of the leukemia inhibitory factor/oncostatin-M (LIF/OSM) family of proteins. The encoded preproprotein is proteolytically processed to generate the mature protein. This protein is a secreted cytokine and growth regulator that inhibits the proliferation of a number of tumor cell lines. This protein also regulates the production of other cytokines, including interleukin 6, granulocyte-colony stimulating factor and granulocyte-macrophage colony stimulating factor in endothelial cells. The OSM protein is consisted of 252 amino acids and its molecular mass is approximately 28.5 kDa.
What is the function of OSM protein?
OSM protein is a member of the interleukin-6 (IL-6) family and has a variety of biological functions, including: OSM can affect cell growth and differentiation. OSM inhibited proliferation in some normal epithelial cells and early tumor cells. In malignant tumor cells, the high expression of OSM was associated with the promotion of cell proliferation. OSM plays an important role in maintaining the hematopoietic microenvironment by regulating G-CSF and SDF-1 to maintain the hematopoietic function of progenitor cells. OSM also plays an important role in the regulation of organ development, tissue injury and regeneration. OSM regulates bone homeostasis, participates in bone remodeling, and may affect the function of osteoblasts and osteoblasts.
OSM Related Signaling Pathway
OSM can bind to the OSM receptor (OSMR) or LIFR (leukocyte suppressor receptor) on the cell surface to form a dimer complex. This binding induces phosphorylation and activation of members of the JAK (Janus kinase) family, which in turn leads to phosphorylation of members of the STAT (signal transduction and transcription-activating protein) family. In addition to the JAK-STAT pathway, OSM also activates the MAPK (mitogen-activated protein kinase) pathway, including ERK (external regulatory protein kinase), JNK (c-Jun amino terminal kinase), and p38 MAPK. PI3K (phosphatidylinositol 3-kinase) is a class of important signal transduction enzymes, and its downstream effector Akt (protein kinase B) plays a key role in many biological processes, such as metabolism, growth, and anti-apoptosis. OSM can activate the PI3K/Akt signaling pathway, thereby affecting cell survival and metabolism. OSM can activate NF-κB signaling pathway and Smad signaling pathway.
OSM Related Diseases
OSM has been linked to the development of a variety of tumors, including liver cancer, melanoma, lung cancer, breast cancer, ovarian cancer, and stomach cancer. It may inhibit cell proliferation in normal epithelial cells and early tumors, and promote cell proliferation in advanced tumors. OSM is involved in bone remodeling and bone metabolism, and is associated with bone-related diseases such as osteoporosis and arthritis. The role of OSM in inflammatory bowel disease suggests that it may regulate the disease process by affecting intestinal inflammatory response and intestinal epithelial barrier function. It is also associated with cardiovascular disease and autoimmune disease.
Bioapplications of OSM
As a cytokine with various biological activities, the study of OSM in a variety of immune diseases and tumors provides the possibility to develop new therapeutic methods. For example, the OSM active protein provided by CUSABIO can be used to study the role of OSM in disease mechanisms or to explore its potential clinical value. Inhibition of OSM may be effective in treating or reducing symptoms, although there is currently a lack of FDA-approved treatments.
Case Study
Case Study 1: Giovanni Di Maira, 2022
Oncostatin M (OSM) is a pleiotropic cytokine of the interleukin (IL)-6 family that contributes to the progression of chronic liver disease. This study investigated the role of OSM in the development and progression of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH). OSM was found to be selectively overexpressed in HCC cells of NAFLD/NASH patients, depending on tumor grade. OSM serum levels, barely detectable in patients with simple steatosis or NASH, were increased in patients with cirrhosis and more evident in those carrying HCC. Cell culture experiments indicated that OSM upregulation in hepatic cancer cells contributes to HCC progression by inducing epithelial-to-mesenchymal transition and increased invasiveness of cancer cells as well as by inducing angiogenesis, which is of critical relevance. In murine xenografts, OSM overexpression was associated with slower tumor growth but an increased rate of lung metastases. Overexpression of OSM and its positive correlation with the angiogenic switch were also confirmed in a murine model of NAFLD/NASH-related hepatocarcinogenesis. Consistent with this, analysis of liver specimens from human NASH-related HCCs with vascular invasion showed that OSM was expressed by liver cancer cells invading hepatic vessels.
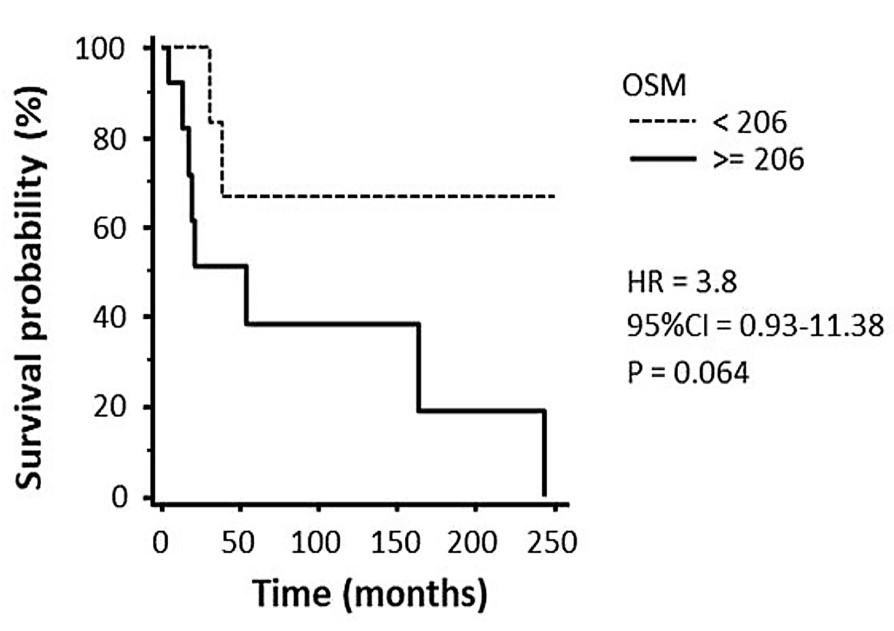
Fig1. Kaplan–Meier curves of survival according to OSM protein levels.
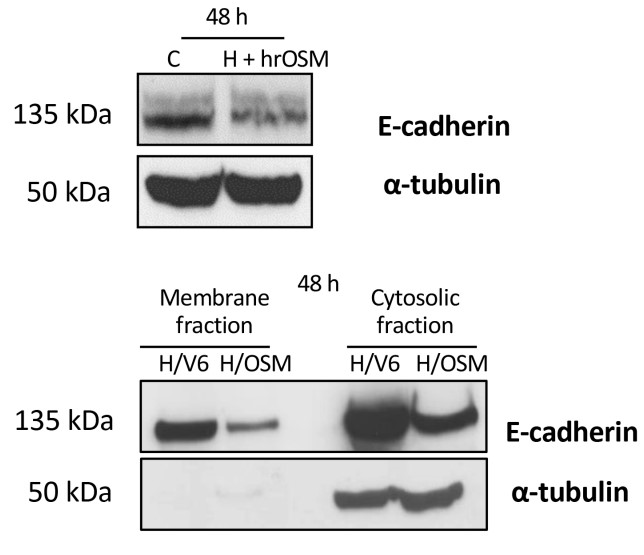
Fig2. Western blotting analysis of E-cadherin expression in total extract obtained from HepG2 naïve cells exposed to hrOSM for 48 h or in membrane and cytosolic fraction extracts from H/OSM cells.
Case Study 2: Simion C Dinca, 2021
The breast tumor microenvironment (TME) is often saturated with proinflammatory cytokines, such as oncostatin M (OSM), which promote epithelial-to-mesenchymal transitions (EMT) in IDC and increased metastasis. Specifically, the reorganization and alignment of collagen fibers in stromal ECM leads to directed tumor cell motility, which promotes metastasis. Lysyl oxidase like-2 (LOXL2) catalyzes ECM remodeling by crosslinking of collagen I in the ECM. This study proposed a novel mechanism whereby OSM induces LOXL2 expression, mediating stromal ECM remodeling of the breast TME. IDC cell lines were treated with OSM (also IL-6, LIF, and IL-1β) and analyzed for LOXL2 expression by qRT-PCR and immunolabelling techniques. Collagen I contraction assays, 3D invasion assays, and confocal microscopy were performed with and without LOXL2 inhibition to determine the impact of OSM-induced LOXL2 on the ECM. The studies demonstrate that human IDC cells treated with OSM resulted in a significant increase in LOXL2 mRNA, which led to upregulated protein expression of secreted, glycosylated, and enzymatically active LOXL2. The expression of LOXL2 in IDC cells did not affect OSM-promoted EMT, and LOXL2 was localized to the cytoplasm and/or secreted. OSM-induced LOXL2 promoted an increase in ECM collagen I fiber crosslinking, which led to significant fiber alignment between cells and increased IDC cell invasion.
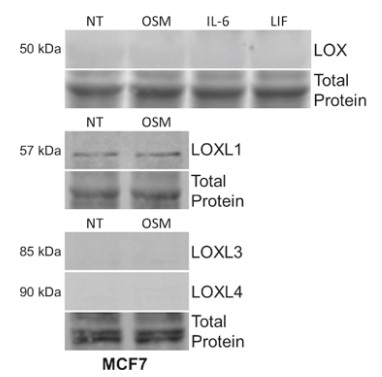
Fig3. MCF7 cells were treated for 24 h with OSM; OSM, IL-6, and LIF for LOX expression.
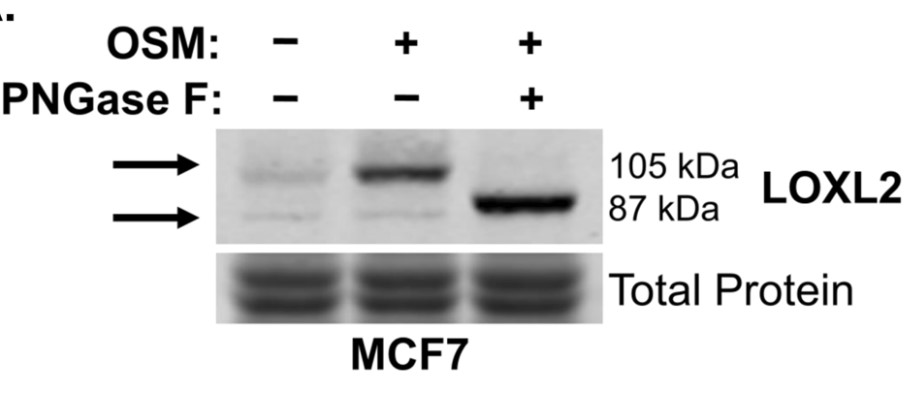
Fig4. OSM-induced LOXL2 is glycosylated, enzymatically active, and secreted from breast cancer cells.
Quality Guarantee
Involved Pathway
OSM involved in several pathways and played different roles in them. We selected most pathways OSM participated on our site, such as Cytokine-cytokine receptor interaction,PIK-Akt signaling pathway,Jak-STAT signaling pathway, which may be useful for your reference. Also, other proteins which involved in the same pathway with OSM were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| PIK-Akt signaling pathway | Fgf15,ITGA2,IFNA8,PTK2,IL7,LPAR5,IL3RA,PPP2R2B,TCL1B,ITGA2B |
| Cytokine-cytokine receptor interaction | CSF2,CXCL2,IL8L1,KITLGB,CCR1L1,IFNE,CCL25B,CCL23,TNFSF13,IL9 |
| Jak-STAT signaling pathway | CCND2A,IFNA16,TSLP,CRLF2,LEPR,IL11,GRB2B,IFNE,PIK3CG,IL21R |
Protein Function
OSM has several biochemical functions, for example, cytokine activity,growth factor activity,oncostatin-M receptor binding. Some of the functions are cooperated with other proteins, some of the functions could acted by OSM itself. We selected most functions OSM had, and list some proteins which have the same functions with OSM. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| growth factor activity | VEGFAA,MIA1,KLK1B4,CLEC11A,CNTF,F2,NDR2,GDF5,VEGFAB,VHL |
| cytokine activity | TNFSF13,CSF3,BMP8B,GDF1,INHBC,TXLNA,BMP6,BMP4,CMTM6,IL11 |
Interacting Protein
OSM has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with OSM here. Most of them are supplied by our site. Hope this information will be useful for your research of OSM.
IL6ST;LIFR;COL4A6;TNNT1
Resources
Related Services
Related Products
References
- Torres, C; Perales, S; et al. Serum Cytokine Profile in Patients With Pancreatic Cancer. PANCREAS 43:1042-1049(2014).
- Lapeire, L; Hendrix, A; et al. Cancer-Associated Adipose Tissue Promotes Breast Cancer Progression by Paracrine Oncostatin M and Jak/STAT3 Signaling. CANCER RESEARCH 74:6806-6819(2014).

.jpg)
.jpg)

