Gastrulation and Germ Layer Formation
Creative BioMart Gastrulation and Germ Layer Formation Product List
Immunology Background
Background
Gastrulation is a key stage in early embryonic development that marks the formation of the three primary germ layers -ectoderm, mesoderm, and endoderm. This process establishes the body plan and is critical for the subsequent differentiation and development of tissues and organs.
The development of a multicellular organism begins with a single cell, the zygote, which undergoes cleavage to form a blastula. Gastrulation, the next critical stage, transforms the simple blastula into a more complex structure and lays the foundation for body patterning through the formation of the three primary germ layers: ectoderm, mesoderm, and endoderm. These germ layers give rise to all the tissues and organs of the adult organism, making gastrulation one of the most important stages of embryonic development. The precise regulation of cell movements, signaling pathways, and gene expression during gastrulation ensures the proper development of the embryo.
Gastrulation: An Overview
Gastrulation is a highly coordinated process of cell movement and differentiation during which the single-layered blastula reorganizes into a multilayered structure with distinct germ layers. The exact mechanisms of gastrulation vary among species, but the fundamental goal remains the same: to establish the body plan and initiate organogenesis.
Initiation of Gastrulation
Gastrulation begins when cells in certain regions of the blastula begin to move inward, forming structures such as the blastopore or primitive streak. In organisms such as amphibians, the process is initiated at the dorsal lip of the blastopore, while in birds and mammals, the primitive streak forms along the midline of the epiblast.
The key movements during gastrulation include:
- Invagination: Cells at a particular region of the blastula fold inward, forming a pocket or indentation.
- Involution: Cells roll inward over an edge, such as the dorsal lip of the blastopore in amphibians.
- Ingression: Individual cells move into the interior of the embryo, as seen in birds and mammals.
- Epiboly: The spreading and thinning of cell layers to envelop the embryo.
These movements contribute to the internalization of cells that will form the mesoderm and endoderm, while the outermost cells remain as the ectoderm.
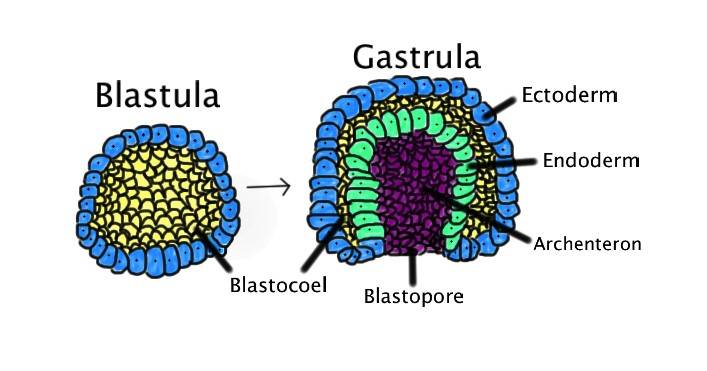 Fig. 1: Process of gastrulation. Gastrulation occurs when a blastula, made up of one layer, folds inward and enlarges to create a gastrula. A gastrula has 3 germ layers--the ectoderm, the mesoderm, and the endoderm. Some of the ectoderm cells from the blastula collapse inward and form the endoderm. The blastospore is the hole created in this action. Whether this blastospore develops into a mouth or an anus determines whether the organism is a protostome or a deuterostome. Ectoderm, blue. Endoderm, green. Blastocoel (the yolk sack), yellow. Archenteron (the gut), purple.
Fig. 1: Process of gastrulation. Gastrulation occurs when a blastula, made up of one layer, folds inward and enlarges to create a gastrula. A gastrula has 3 germ layers--the ectoderm, the mesoderm, and the endoderm. Some of the ectoderm cells from the blastula collapse inward and form the endoderm. The blastospore is the hole created in this action. Whether this blastospore develops into a mouth or an anus determines whether the organism is a protostome or a deuterostome. Ectoderm, blue. Endoderm, green. Blastocoel (the yolk sack), yellow. Archenteron (the gut), purple.Gastrulation Event and Regulation
Gastrulation, which occurs during the third week of embryonic development, is a critical event that establishes the three germ layers -ectoderm, mesoderm, and endoderm - through the migration of epiblast cells across the primitive streak. These layers later give rise to all major tissues and organs, marking the beginning of organogenesis. In addition, gastrulation establishes the major body axes: the anteroposterior (AP), dorsoventral (DV), and left-right (LR) axes, which are essential for proper body patterning.
The molecular regulation of gastrulation is controlled by several key signaling pathways and molecules. Nodal, a member of the TGF-β family, plays a central role in mesoderm and endoderm formation as well as the establishment of the AP axis. Its expression is tightly controlled by feedback loops involving antagonists such as Lefty and Cerberus, which ensure spatial and temporal precision.
- BMP (Bone Morphogenetic Protein) signaling (BMP2, BMP10) is critical for DV axis specification and regulates the differentiation of mesodermal tissues. BMP activity is modulated by antagonists such as Noggin and Chordin, which create a gradient that defines dorsal and ventral regions.
- FGF (Fibroblast Growth Factor) signaling contributes to mesoderm patterning and cell movement during gastrulation by promoting epithelial-mesenchymal transition (EMT), which is essential for cells to migrate through the primitive streak.
- Wnt signaling, particularly canonical Wnt/β-catenin, is involved in AP axis formation and regulation of mesoderm and ectoderm development. Shh (Sonic Hedgehog) signaling contributes to the establishment of the LR axis and proper organ symmetry.
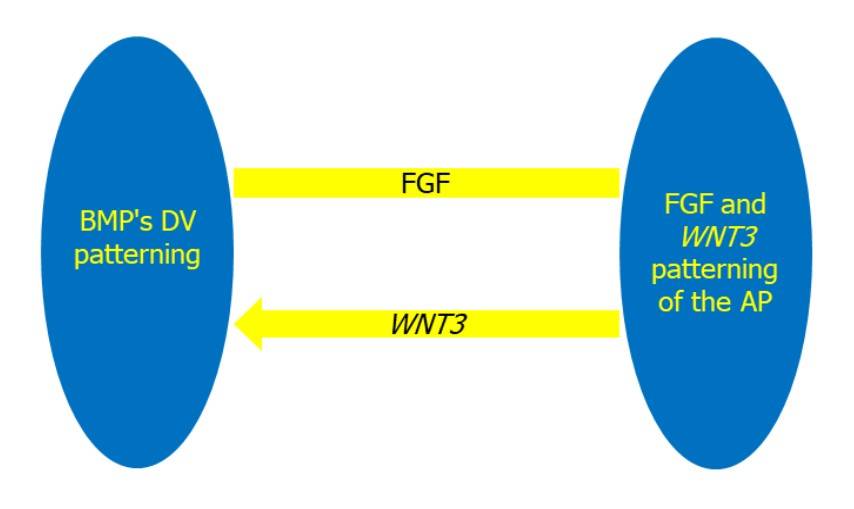 Fig. 2: The establishment of the DV and AP axes is controlled by interconnected regulatory mechanisms. The DV axis is determined by BMP signaling, which induces ventral fate, whereas the FGF and WNT3 signaling pathways control AP patterning. WNT3 stimulation, on the other hand, also promotes BMP signaling (Abas et al., 2022).
Fig. 2: The establishment of the DV and AP axes is controlled by interconnected regulatory mechanisms. The DV axis is determined by BMP signaling, which induces ventral fate, whereas the FGF and WNT3 signaling pathways control AP patterning. WNT3 stimulation, on the other hand, also promotes BMP signaling (Abas et al., 2022).Disruptions in any of these pathways can lead to gastrulation abnormalities resulting in congenital diseases such as neural tube defects, situs inversus, or mesodermal dysplasia, highlighting the critical importance of precise molecular control during this phase of development.
Germ Layer Formation
Once gastrulation is initiated, the three primary germ layers -ectoderm, mesoderm, and endoderm - are formed. Each germ layer gives rise to specific tissues and organs, and their proper formation is essential for normal development.
Ectoderm
The ectoderm is the outermost germ layer and gives rise to the epidermis, nervous system, and sensory organs. It plays a critical role in forming the central and peripheral nervous systems, including the brain and spinal cord.
- Neurulation: A process closely linked to gastrulation, neurulation involves the formation of the neural tube from the ectoderm, which later develops into the central nervous system. The neural plate forms from a specialized region of the ectoderm and folds inward to create the neural tube.
- Epidermis and Sensory Organs: The remaining ectoderm differentiates into the epidermis, which forms the skin, hair, and nails, as well as sensory organs such as the eyes and ears.
The regulation of ectodermal differentiation is controlled by factors such as BMP signaling, which promotes epidermal fate, while inhibition of BMP is required for neural differentiation.
Mesoderm
The mesoderm is the middle germ layer and gives rise to a wide range of tissues, including muscles, bones, the cardiovascular system, and connective tissues. Mesodermal cells are highly migratory and contribute to various structures during development.
- Somites and Skeletal Muscle: The paraxial mesoderm forms somites, which give rise to the vertebral column, skeletal muscles, and dermis of the skin.
- Cardiovascular System: The lateral plate mesoderm forms the heart and blood vessels. The early specification of cardiac progenitor cells is regulated by FGF and Nodal signaling.
- Urogenital System: The intermediate mesoderm gives rise to the kidneys, gonads, and their associated structures.
The precise differentiation of mesodermal derivatives depends on gradients of signaling molecules such as Nodal, Wnt, and BMP, which establish distinct regions of the mesoderm along the embryonic axis.
Endoderm
The endoderm is the innermost germ layer and gives rise to the lining of the digestive and respiratory tracts, as well as associated organs such as the liver and pancreas.
- Digestive Tract: The endoderm forms the epithelial lining of the gastrointestinal tract, including the stomach, intestines, and esophagus. The development of these structures is regulated by signals from the surrounding mesoderm and ectoderm.
- Respiratory System: The endoderm also forms the lining of the respiratory tract, including the lungs and trachea. FGF signaling from the surrounding mesoderm promotes the branching morphogenesis required for lung development.
- Liver and Pancreas: The endoderm gives rise to the liver and pancreas through the differentiation of specific progenitor cells in response to local signaling cues such as Hedgehog and FGF.
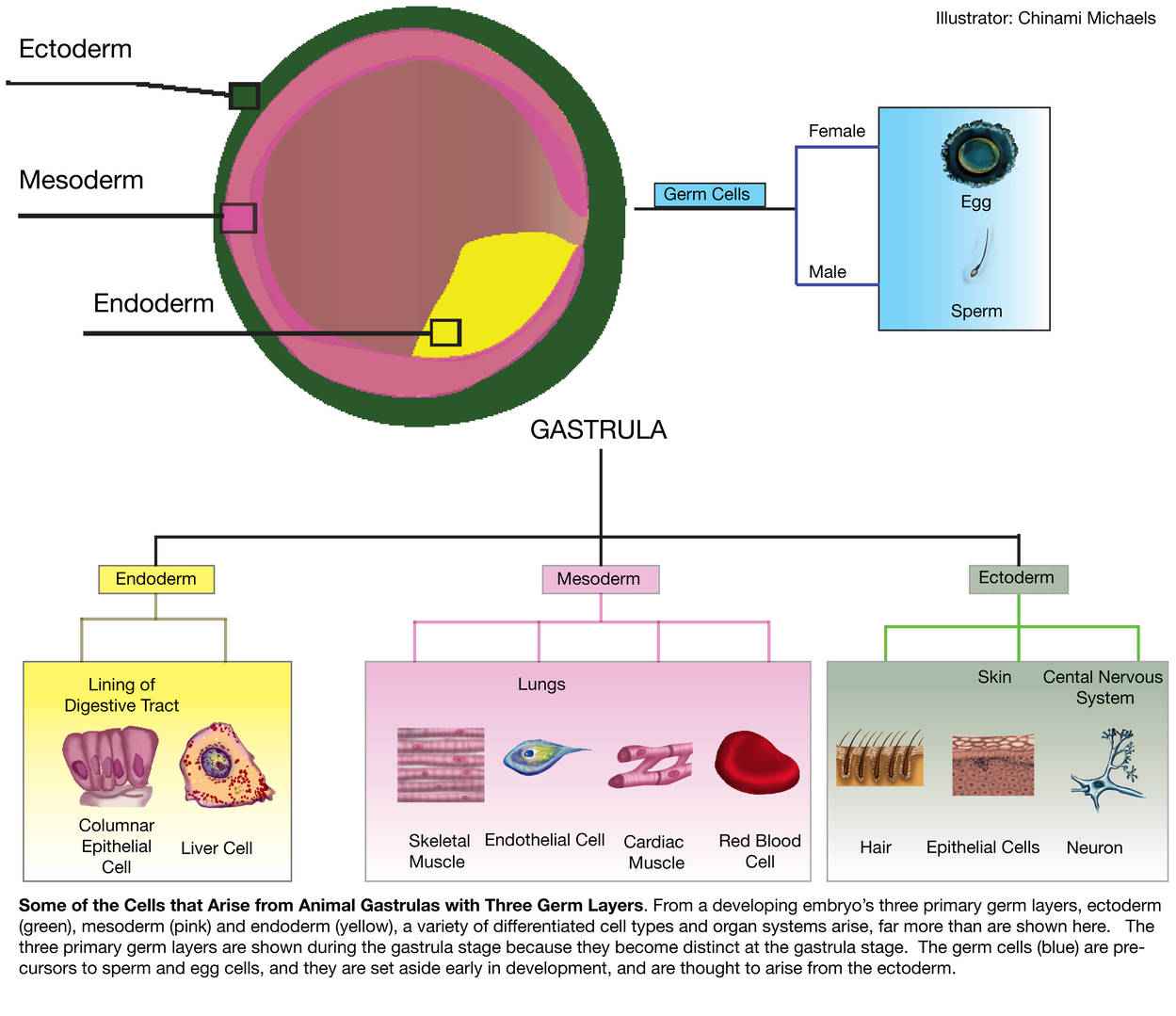 Fig. 3: Cells and tissues that develop from endoderm, mesoderm and ectoderm (Michaels, 2014).
Fig. 3: Cells and tissues that develop from endoderm, mesoderm and ectoderm (Michaels, 2014).Gastrulation Across Species
Although the fundamental aspects of gastrulation and germ layer formation are conserved across species, there are notable differences in the specific mechanisms and morphologies.
Amphibians
In amphibians such as frogs, gastrulation begins with the formation of the dorsal lip of the blastopore. Cells from the surface involute over the lip and migrate inward to form the mesoderm and endoderm. The ectoderm remains on the outside. The Spemann-Mangold organizer plays a critical role in directing these movements and patterning the embryo.
Birds
In birds, gastrulation occurs through the formation of the primitive streak, a structure that defines the future anterior-posterior axis of the embryo. Cells enter through the primitive streak to form the mesoderm and endoderm, while the ectoderm remains on the surface.
Mammals
In mammals, gastrulation is similar to that in birds, with the formation of a primitive streak in the epiblast. However, the presence of a trophoblast (which forms the placenta) and the development of an amnion add complexity to the process. In humans, gastrulation occurs around day 14 of development.
Early Lineage Markers in Embryonic Development
During early embryogenesis, the specification of the three primary germ layers -ectoderm, mesoderm and endoderm - is a critical event that sets the stage for the differentiation of all tissues and organs. The identification of early lineage markers for each germ layer allows researchers to track the development of specific cell types and to understand the signaling pathways that regulate lineage commitment. Early lineage markers are proteins or genes that are expressed in cells as they begin to differentiate into their respective germ layers. This section introduces the major early lineage markers for the ectoderm, mesoderm, and endoderm.
Early Ectodermal Lineage Markers
The ectoderm is the outermost germ layer, responsible for forming the nervous system, epidermis, and sensory organs. Several transcription factors and signaling molecules are associated with the early specification of ectodermal cells.
- Sox2: Sox2 is a transcription factor crucial for maintaining the pluripotency of embryonic stem cells and is one of the earliest markers of neural ectoderm differentiation. It plays a key role in the development of the central nervous system, particularly in specifying neural progenitors.
- Pax6: Pax6 is a transcription factor involved in the development of the eyes and central nervous system. It is one of the earliest markers of neural ectoderm and is essential for the formation of neural tissues, particularly in the forebrain and eyes.
- Keratin 8/18 (K8/K18): These cytokeratins are early markers of surface ectoderm and are associated with the differentiation of epithelial cells that will give rise to the epidermis, hair, and nails.
- Nestin: Nestin is an intermediate filament protein expressed in neural progenitor cells. It is commonly used as an early marker of neuroectoderm differentiation, particularly in the development of the brain and spinal cord.
- N-cadherin: N-cadherin is a cell adhesion molecule that is involved in neural tube formation. Its expression is indicative of cells undergoing neurulation, marking their commitment to the neural ectoderm lineage.
Early Endodermal Lineage Markers
The endoderm is the innermost germ layer and gives rise to the epithelial lining of the digestive and respiratory tracts, as well as associated organs such as the liver and pancreas. Several transcription factors and proteins are recognized as early markers of endoderm differentiation.
- Sox17: Sox17 is a transcription factor critical for the early specification of endodermal cells. It is involved in the development of the definitive endoderm, which forms the epithelial linings of the gastrointestinal and respiratory systems.
- FoxA2 (HNF-3β): FoxA2 is a pioneer transcription factor essential for early endoderm formation. It regulates the differentiation of cells into the gut endoderm and is involved in the development of several organs, including the liver and pancreas.
- GATA4: GATA4 is a transcription factor that plays a key role in early endoderm differentiation, particularly in the formation of the heart, lungs, and liver. It is also involved in the mesoderm-endoderm interaction during organogenesis.
- C-X-C chemokine receptor type 4 (CXCR4): CXCR4 is a cell surface receptor that is expressed on early endoderm progenitors. It plays a role in guiding endodermal cells to their proper location during embryogenesis and is involved in the development of the pancreas and liver.
- Alpha-fetoprotein (AFP): AFP is a protein produced by the yolk sac and fetal liver, and it is an early marker of hepatocyte differentiation. Its expression is indicative of endodermal lineage commitment, especially in the liver and gastrointestinal development.
Early Mesodermal Lineage Markers
The mesoderm is the middle germ layer that gives rise to muscles, bones, the cardiovascular system, and connective tissues. The commitment of cells to the mesodermal lineage is marked by the expression of several key proteins and transcription factors.
- Brachyury (T): Brachyury is a transcription factor that is one of the earliest markers of mesoderm differentiation. It plays a central role in the development of the notochord and is essential for the formation of the posterior mesoderm. Brachyury is also involved in the movement and differentiation of mesodermal progenitors during gastrulation.
- Mesoderm posterior 1 (MESP1): MESP1 is a transcription factor that plays a critical role in the early specification of mesodermal cells, particularly in the formation of the cardiovascular system. It is considered one of the earliest markers of cardiac mesoderm differentiation.
- Mixl1: Mixl1 is a homeobox transcription factor involved in mesoderm formation and patterning. It is expressed in the primitive streak and is essential for the differentiation of mesodermal tissues, particularly in the development of the heart and muscles.
- T-box transcription factor TBX6: TBX6 is a transcription factor that is crucial for paraxial mesoderm differentiation, which gives rise to somites and the axial skeleton. TBX6 is involved in somitogenesis and plays a role in the segmentation of the vertebral column.
- Nkx2.5: Nkx2.5 is a transcription factor specifically involved in early cardiac mesoderm differentiation. It is expressed in progenitor cells that give rise to the heart and is essential for proper heart formation and function.
- Flk1 (VEGFR2): Flk1, also known as Vascular Endothelial Growth Factor Receptor 2 (VEGFR2), is a receptor tyrosine kinase expressed in early mesodermal cells. It is involved in the formation of blood vessels (vasculogenesis) and hematopoietic tissues during mesoderm development.
Gastrulation and germ layer formation are essential processes in embryonic development, marking the transition from a simple blastula to a complex, multi-layered embryo with distinct tissue types. The proper coordination of cell movements, signaling pathways, and gene expression during gastrulation ensures the formation of the three primary germ layers, each of which contributes to specific tissues and organs. Understanding these processes is critical for insights into congenital disorders, stem cell biology, and regenerative medicine, as disruptions in gastrulation can lead to significant developmental defects.
Case Study
Case 1: Pereira, L. A.; et al. Brachyury and related Tbx proteins interact with the Mixl1 homeodomain protein and negatively regulate mixl1 transcriptional activity. PLOS ONE. 2011, 6(12), e28394.
Mixl1 is a homeodomain transcription factor essential for mesoderm and endoderm patterning during mammalian embryogenesis. Although Mixl1 plays a critical role in development, its cofactors remain largely unknown. In this study, the researchers show that Mixl1 activity is modulated by protein-protein interactions, with T-box factors acting as negative regulators of Mixl1 function.
To investigate the functional consequences of the Mixl1Brachyury interaction, they used activation of transcription of the Gsc promoter as an assay for Mixl1 transcriptional activation. Results showed that co-expression of Brachyury repressed Mixl1-induced activation of the Gsc promoter in a dose-dependent manner. Deletion mutant analysis revealed that neither the T-box domain alone nor a Brachyury mutant lacking the T-box domain repressed Mixl1 activity. Full-length T-box proteins, such as Eomes and Tbx6, also inhibited Mixl1's activity on the Gsc and Pdgfra promoters.
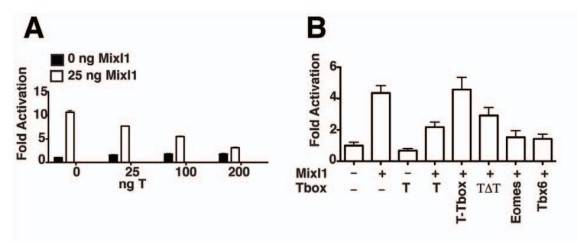 Fig. 4: T and related Tbx factors reduce Mixl1 induction of the Gsc promoter. (A) Luciferase assays showing the effect of T on the transactivation activity of Mixl1. C2C12 cells were co-transfected with 100 ng of a pGL3 Gsc promoter-luciferase reporter construct, 25 ng pMT2-HAMixl1 and increasing amounts of pMT2-HA-T as indicated. Regression analysis indicates that increasing amounts of T correlates with a significant decrease in Mixl1 transactivation, p<0.001. (B) The effect of T domains and related Tbx members on the transactivation activity of Mixl1. Luciferase Reporter analysis was performed as in (A) with 25 ng pMT2-HA-Mixl1 and 200 ng of pMT2-HA-T, T-Tbox, T ΔTbox (TΔT), Eomes or Tbx6. For A and B, results from an independent experiment are shown. Error bars show the S.E.M., n = 3, p<0.05 (Mixl1 vs Mixl1 co-transfected with T), p>0.05 (Mixl1 vs Mixl1 co-transfected with T-Tbox or TΔT), p<0.01 (Mixl1 vs Mixl1 co-transfected with Eomes or Tbx6).
Fig. 4: T and related Tbx factors reduce Mixl1 induction of the Gsc promoter. (A) Luciferase assays showing the effect of T on the transactivation activity of Mixl1. C2C12 cells were co-transfected with 100 ng of a pGL3 Gsc promoter-luciferase reporter construct, 25 ng pMT2-HAMixl1 and increasing amounts of pMT2-HA-T as indicated. Regression analysis indicates that increasing amounts of T correlates with a significant decrease in Mixl1 transactivation, p<0.001. (B) The effect of T domains and related Tbx members on the transactivation activity of Mixl1. Luciferase Reporter analysis was performed as in (A) with 25 ng pMT2-HA-Mixl1 and 200 ng of pMT2-HA-T, T-Tbox, T ΔTbox (TΔT), Eomes or Tbx6. For A and B, results from an independent experiment are shown. Error bars show the S.E.M., n = 3, p<0.05 (Mixl1 vs Mixl1 co-transfected with T), p>0.05 (Mixl1 vs Mixl1 co-transfected with T-Tbox or TΔT), p<0.01 (Mixl1 vs Mixl1 co-transfected with Eomes or Tbx6).Case 2: Costello, I.; et al. The T-box transcription factor Eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nature Cell Biology. 2011, 13(9), 1084–1091.
This study highlights the role of the T-box transcription factor Eomesodermin (Eomes) as a key regulator in early embryonic development. Eomes is essential for processes like epithelial-to-mesenchymal transition, mesoderm migration, and definitive endoderm specification during gastrulation. The research reveals that Eomes expression in the primitive streak marks the earliest cardiac mesoderm and promotes cardiovascular progenitor formation by directly activating the transcription factor gene Mesp1, upstream of core cardiac transcriptional machinery. Unlike its interaction with Nodal signaling in endoderm formation, cardiac progenitor development requires only low levels of Nodal activity via a Foxh1/Smad4-independent pathway. Overall, Eomes directs distinct transcriptional programs for specifying cardiac and endoderm progenitors during gastrulation.
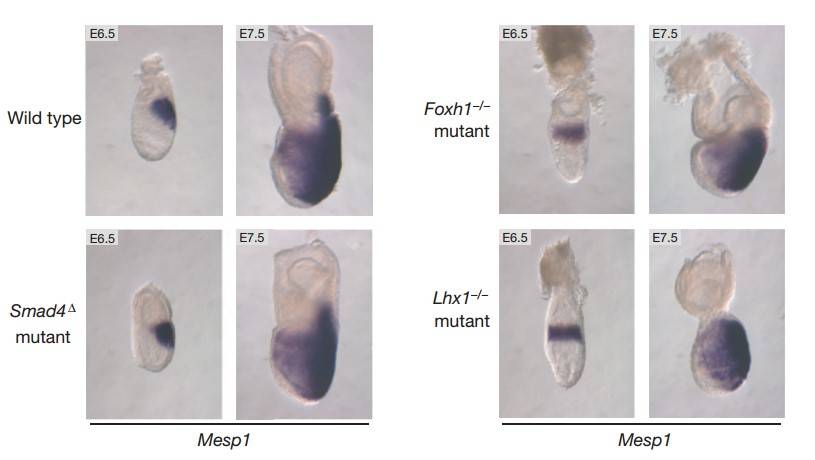 Fig. 5: Eomes and dose-dependent Nodal/Smad2/3 signaling levels control cardiac mesoderm and definitive endoderm specification during gastrulation. (a) Smad4 and Foxh1 are critical Nodal pathway components for transducing high levels of signaling. Mesp1 is expressed normally in E6.5 and E7.5 Foxh1-null embryos and in embryos lacking Smad4 in the epiblast only (Smad 4Δ). Mesp1 expression is also efficiently induced in Lhx1-mutant embryos, which show definitive endoderm and midline mesoderm defects. However, the failure of anterior–posterior axis rotation results in induction of Mesp1 throughout the proximal epiblast.
Fig. 5: Eomes and dose-dependent Nodal/Smad2/3 signaling levels control cardiac mesoderm and definitive endoderm specification during gastrulation. (a) Smad4 and Foxh1 are critical Nodal pathway components for transducing high levels of signaling. Mesp1 is expressed normally in E6.5 and E7.5 Foxh1-null embryos and in embryos lacking Smad4 in the epiblast only (Smad 4Δ). Mesp1 expression is also efficiently induced in Lhx1-mutant embryos, which show definitive endoderm and midline mesoderm defects. However, the failure of anterior–posterior axis rotation results in induction of Mesp1 throughout the proximal epiblast.References
- Abas, R., Masrudin, S. S., Harun, A. M., & Omar, N. S. (2022). Gastrulation and body axes formation: A molecular concept and its clinical correlates. The Malaysian Journal of Medical Sciences: MJMS, 29(6), 6.
- Costello, I., Pimeisl, I.-M., Dräger, S., Bikoff, E. K., Robertson, E. J., & Arnold, S. J. (2011). The T-box transcription factor Eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nature Cell Biology, 13(9), 1084–1091.
- Michaels, C., (2014). Some of the Cells that Arise from Animal Gastrulas with Three Germ Layers. Embryo Project Encyclopedia. ISSN: 1940-5030.
- Pereira, L. A., Wong, M. S., Lim, S. M., Sides, A., Stanley, E. G., & Elefanty, A. G. (2011). Brachyury and related tbx proteins interact with the mixl1 homeodomain protein and negatively regulate mixl1 transcriptional activity. PLOS ONE, 6(12), e28394.

