TTR
-
Official Full Name
transthyretin -
Overview
This gene encodes transthyretin, one of the three prealbumins including alpha-1-antitrypsin, transthyretin and orosomucoid. Transthyretin is a carrier protein; it transports thyroid hormones in the plasma and cerebrospinal fluid, and also transports retinol (vitamin A) in the plasma. The protein consists of a tetramer of identical subunits. More than 80 different mutations in this gene have been reported; most mutations are related to amyloid deposition, affecting predominantly peripheral nerve and/or the heart, and a small portion of the gene mutations is non-amyloidogenic. The diseases caused by mutations include amyloidotic polyneuropathy, euthyroid hyperthyroxinaemia, amyloidotic vitreous opacities, cardiomyopathy, oculoleptomeningeal amyloidosis, meningocerebrovascular amyloidosis, carpal tunnel syndrome, etc. [provided by RefSeq, Jan 2009] -
Synonyms
TTR;transthyretin;CTS;CTS1;PALB;TBPA;HEL111;HsT2651;ATTR;carpal tunnel syndrome 1;thyroxine-binding prealbumin;epididymis luminal protein 111;prealbumin, amyloidosis type I
Recombinant Proteins
- Human
- Mouse
- Cynomolgus
- Zebrafish
- Rhesus macaque
- Chicken
- Rat
- Cattle
- Dog
- Guinea pig
- Pig
- Rabbit
- Macaca Fascicularis
- Sheep
- Bovine
- Pongo pygmaeus abelii
- HEK293
- Trichoplusia Ni Larval
- E.coli
- Mammalian Cells
- Human Cells
- Yeast
- Human Amniotic
- Sheep Serum
- Bovine Serum
- Human Plasma
- His
- Non
- Myc
- T7
- Trx
- Flag
- Avi
- Fc
- DDK
- SUMO
- GST
Background
What is TTR protein?
TTR gene (transthyretin) is a protein coding gene which situated on the long arm of chromosome 18 at locus 18q12. This gene encodes one of the three prealbumins, which include alpha-1-antitrypsin, transthyretin and orosomucoid. The encoded protein, transthyretin, is a homo-tetrameric carrier protein, which transports thyroid hormones in the plasma and cerebrospinal fluid. It is also involved in the transport of retinol (vitamin A) in the plasma by associating with retinol-binding protein. The protein may also be involved in other intracellular processes including proteolysis, nerve regeneration, autophagy and glucose homeostasis. The TTR protein is consisted of 147 amino acids and TTR molecular weight is approximately 15.9 kDa.
What is the function of TTR protein?
TTR protein, also known as transthyroxine, is a highly conserved tetramer transport protein found in vertebrates. It is synthesized in the liver, choroid plexus, and retinal pigment epithelial cells, and secreted into the blood, cerebrospinal fluid, and eyes. TTR's main function is to transport the thyroid hormone thyroxine and retinol-binding protein (RBP, the transporter of vitamin A). In addition to its function as a transport protein, TTR's potential role in neurobiology and disease pathophysiology is also being investigated, including its potential role in neuroprotection and promotion of nerve regeneration.
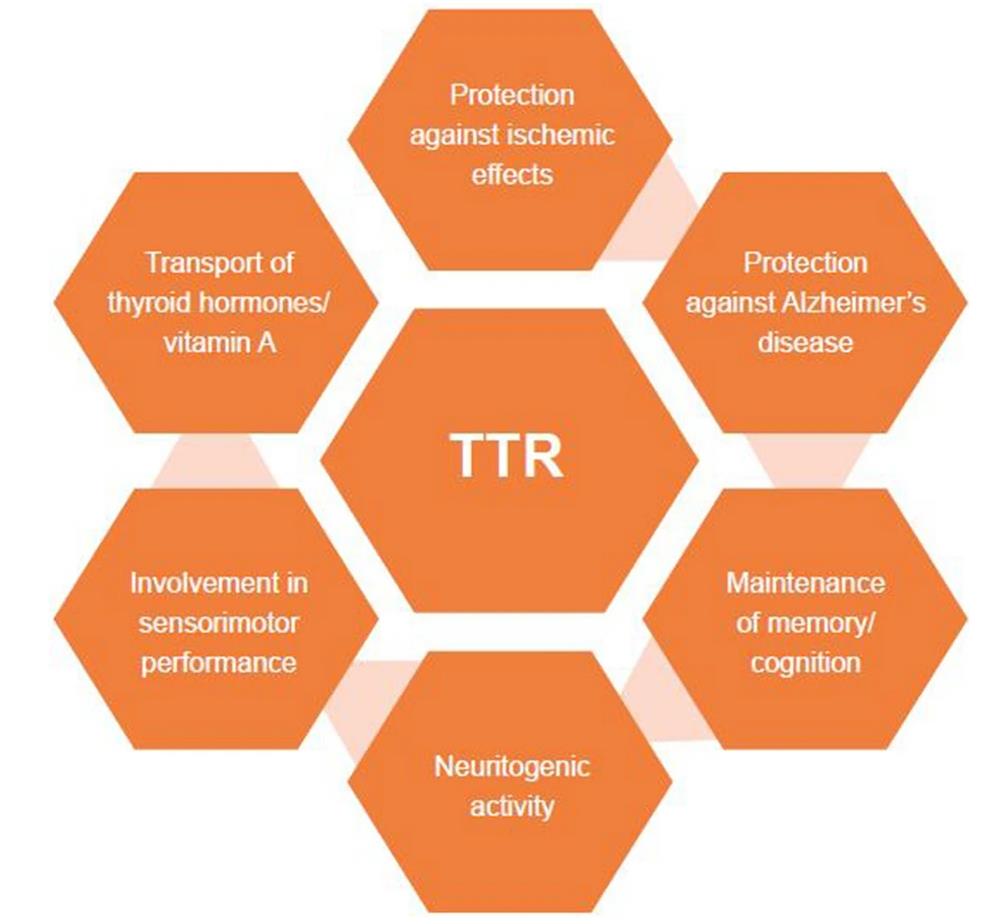
Fig1. Known biological functions of TTR from mouse models and human studies. (Marcia Almeida Liz, 2020)
TTR related signaling pathway
Signaling pathways associated with TTR protein (transthyroxin protein) are mainly involved in its transport function in the body and amyloidosis under specific conditions. Under normal circumstances, TTR exists as A tetramer and is responsible for transporting thyroid hormones and retinol-binding protein-vitamin A complexes. However, in hereditary transthyroxin protein amyloidosis (hATTR), abnormal aggregation of TTR forms amyloid plaques that lead to impaired nervous system and organ function. In this process, TTR protein dissociation, misfolding and deposition are key signaling events, which may involve signaling pathways including protein homeostasis networks, endocytosis and autophagy, and interactions with extracellular matrix.
TTR related diseases
TTR protein (transthyroxin protein) related diseases mainly include hereditary transthyroxin protein amyloidosis (hATTR), a disease caused by mutations in the TTR gene that is characterized by the accumulation of abnormal amyloid protein in body organs and tissues, especially the heart and peripheral nerves. Symptoms resulting from this accumulation include peripheral sensorimotor neuropathy, autonomic neuropathy, and cardiomyopathy. ATTR is further divided into inherited type (ATTRm) and wild type (ATTRwt), wherein ATTRm is an autosomal dominant genetic disease, while ATTRwt mainly affects people over the age of 60. Attr-cm (transthyroxin amyloid cardiomyopathy) is a form of ATTR with a poor prognosis and a life expectancy of only 2 to 6 years.
Bioapplications of TTR
Recombinant human transthyroxin protein (rhTTR) has a variety of applications in the biomedical field, mainly including as a research tool to understand the pathological mechanism of TTR related diseases such as hereditary transthyroxin protein amyloidosis (hATTR). rhTTR is also used to develop and evaluate therapeutic strategies for these diseases, for example by using rhTTR to screen for small molecule drugs that can reduce TTR protein deposition or stabilize its structure. In addition, rhTTR is used as a target protein in drug development to assist in the research and design of therapies targeting abnormal function of TTR, including gene silencing techniques such as RNA interference (RNAi) and gene editing techniques. These studies and applications drive understanding and innovation in the treatment of diseases such as hATTR, providing patients with new treatment options.
Case Study
Case Study 1: Bandar Alshehri, 2020
Transthyretin (TTR), known for its role in transporting thyroid hormones, was studied in wildtype and TTR null mice to understand its impact on brain development. Contrary to expectations of hypothyroidism in TTR null mice, researchers found a hypermyelination phenotype with increased oligodendrocyte density, enhanced proliferation and migration of oligodendrocyte precursor cells (OPCs), and reduced apoptosis. TTR null mice also showed TTR synthesis in OPCs, indicating an additional function beyond hormone distribution. The hypermyelination might be linked to increased pAKT, which is crucial for oligodendrocyte maturation. Understanding TTR's role in neural stem cells and OPCs could inform treatments for demyelinating diseases.
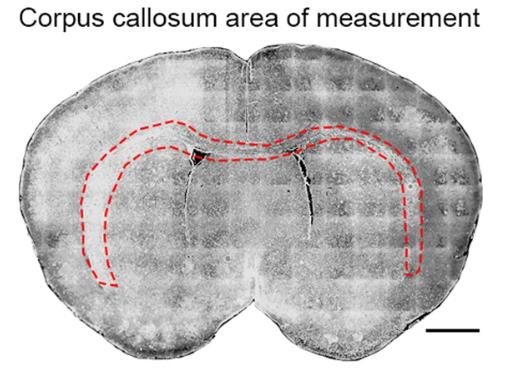
Fig1. Measured area outlining the corpus callosum used in cell density analyses of Olig2, CC1 and DAPI positive cells from wild type and TTR null mice.
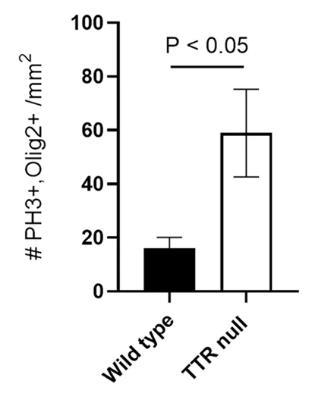
Fig2. A semi-quantitative cell density analysis of double positive PH3/Olig2 cells counted per mm2 from P4 wild type and TTR null mice.
Case Study 2: Marcus Jäger, 2024
Proteins like transthyretin (TTR), which has a long lifespan in the bloodstream and cerebrospinal fluid, are known for their kinetic stability. This study introduces a rate-expansion method to measure unfolding rates, showing that Escherichia coli TTR-related proteins (EcTRP) possess this stability despite being more structurally frustrated and mutation-sensitive than human TTR. A mutational analysis reveals that tyrosine 111 in EcTRP, replaced by threonine in TTR, is key to reducing frustration. TTR's evolution involved compensatory changes to preserve kinetic stability and reduce mutation sensitivity, proposing an evolutionary pathway from ancestral enzymes to modern TTRs.
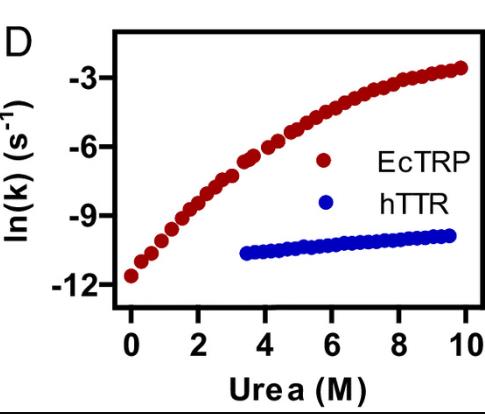
Fig3. The rate plot of hTTR differs significantly from the rate plot of EcTRP, and is consistent with simple two-state unfolding of a minimally frustrated protein.
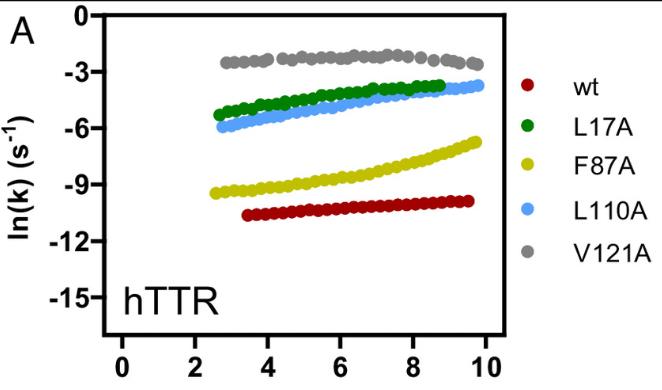
Fig4. Kinetic analysis of hTTR variants that carry mutations.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (TTR-728H)
.
.jpg)
Fig2. SDS-PAGE (TTR-3880H)
Involved Pathway
TTR involved in several pathways and played different roles in them. We selected most pathways TTR participated on our site, such as Amyloid formation,Disease,Diseases associated with visual transduction, which may be useful for your reference. Also, other proteins which involved in the same pathway with TTR were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| FOXA2 and FOXA3 transcription factor networks | FOXA1,FOXF1,CPT1B,UCP2,CEBPA,KCNJ11,CPT1A,CPT1C,DLK1 |
| Extracellular matrix organization | COL25A1,CMA1,COL6A3,LTBP3,DMP1,COL11A1B,MFAP1,MMP13,COL4A4,COL6A1 |
| Diseases associated with visual transduction | STRA6,RLBP1,RBP1,OPN1MW2,RBP4,OPN1LW,OPN1SW,OPN1MW |
| Disease | ANTXR1,TCEA1,TNKS2,NUP35,CDK9,RBP4,NUPL2,AP2B1,LRRFIP1,TRAT1 |
| Diseases of signal transduction | CTBP1,RBP4,TNKS,TRIM24,RLBP1,CDK8,TNKS2,CCNC,TRAT1,STRA6 |
| Amyloid formation | NAT8B,RHBDL1,HIST3H2BB,ODAM,HIST2H3C,APCS,SEMG1,TGFBI,NAT8,CEACAM21 |
| Non-integrin membrane-ECM interactions | TRAPPC4,DDR2,DMD,DDR1,LAMC3 |
| Metabolism of proteins | ADAMTS18,FXC1,ARL2,SFTPBB,SEC23IP,B3GNT8,TRAPPC1,LMCD1,ZNF408,ANKRD28 |
Protein Function
TTR has several biochemical functions, for example, hormone activity,hormone binding,identical protein binding. Some of the functions are cooperated with other proteins, some of the functions could acted by TTR itself. We selected most functions TTR had, and list some proteins which have the same functions with TTR. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| hormone activity | UTS2A,PYY,SST1.1,GCGA,SST3,INS,CSH1,RLN2,IGF1,NPPB |
| protein binding | PPP1R13L,ZNF187,BCL2L15,PES1,NPHS1,MED8,ZNF502,EMD,ZNF136,BRCA1 |
| protein heterodimerization activity | APOA2,JAM3,NKX2-5,DRD1A,OSTB,CEBPB,TENM2,ZBTB1,FLOT1,THRA |
| hormone binding | MC5R,GPR30,MC1R,MTNR1A,NPR3,NPR2,AMHR2,CALR,UCN2,CRYM |
| identical protein binding | P2RX2,TGFB3,PSMA7,MAP1S,SH3GL3,CUBN,STXBP1,COL2A1,LDHA,CLDN9 |
Interacting Protein
TTR has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with TTR here. Most of them are supplied by our site. Hope this information will be useful for your research of TTR.
MT3;NECAB2;CDR2;AGER;IKBKAP;VIM
Resources
Related Services
Related Products
References
- Haussecker, D; et al. Current issues of RNAi therapeutics delivery and development. JOURNAL OF CONTROLLED RELEASE 195:49-54(2014).
- Obici, L; Merlini, G; et al. An overview of drugs currently under investigation for the treatment of transthyretin-related hereditary amyloidosis. EXPERT OPINION ON INVESTIGATIONAL DRUGS 23:1239-1251(2014).


