Prnp
-
Official Full Name
prion protein -
Overview
The protein encoded by this gene is a membrane glycosylphosphatidylinositol-anchored glycoprotein that tends to aggregate into rod-like structures. The encoded protein contains a highly unstable region of five tandem octapeptide repeats. This gene is found on chromosome 20, approximately 20 kbp upstream of a gene which encodes a biochemically and structurally similar protein to the one encoded by this gene. Mutations in the repeat region as well as elsewhere in this gene have been associated with Creutzfeldt-Jakob disease, fatal familial insomnia, Gerstmann-Straussler disease, Huntington disease-like 1, and kuru. Alternative splicing results in multiple transcript variants encoding the same protein. -
Synonyms
PRNP;CJD;GSS;PrP;ASCR;PRIP;PrPc;CD230;prion;MGC26679;PrP27-30;PrP33-35C;prion protein;major prion protein;p27-30;CD230 antigen;prion protein PrP;OTTHUMP00000030161;OTTHUMP00000030162;OTTHUMP00000030163;OTTHUMP00000161139;prion-related protein;prion protein (p27-30) (Creutzfeldt-Jakob disease, Gerstmann-Strausler-Scheinker syndrome, fatal familial insomnia)
Recombinant Proteins
- Human
- Cervid
- Mouse
- Rhesus macaque
- Hamster
- Bovine
- Rat
- Sheep
- Chicken
- Ovine
- Cynomolgus
- Bos
- Golden hamster
- Cervus elaphus
- HEK293
- E.coli
- Mammalian Cells
- Human Cells
- Yeast
- His
- Non
- Fc
- hIgG4
- Avi
- Myc
Background
What is PRNP protein?
PRNP gene (prion protein) is a protein coding gene which situated on the short arm of chromosome 20 at locus 20p13. The protein encoded by this gene is a membrane glycosylphosphatidylinositol-anchored glycoprotein that tends to aggregate into rod-like structures. The encoded protein contains a highly unstable region of five tandem octapeptide repeats. It is most richly expressed in the central nervous system and has been linked to a rare neurodegenerative disease called prion disease. Studies have shown that it may be involved in cell signaling, cell adhesion and apoptosis. The PRNP protein is consisted of 73 amino acids and PRNP molecular weight is approximately 8.7 kDa.
What is the function of PRNP protein?
The PRNP protein is a cell-surface glycoprotein with a yet-to-be fully understood physiological function, but it is thought to be involved in processes such as cell signaling, cell adhesion, and apoptosis. While its normal role remains elusive, the protein's abnormal isoform is associated with prion diseases, highlighting the importance of understanding its function for both basic neuroscience and potential therapeutic interventions.
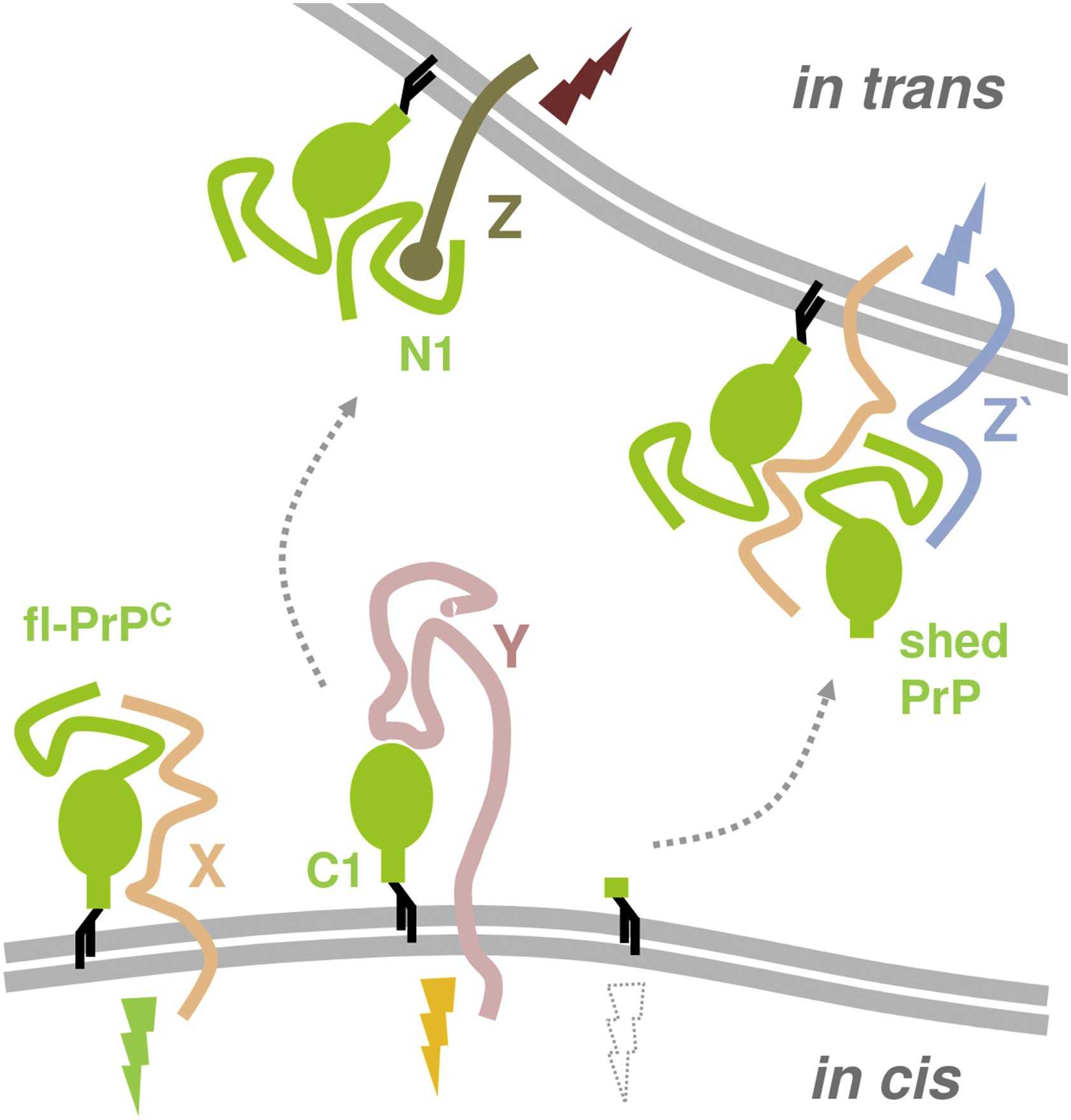
Fig1. PrPC cleavages influence protein interactions and functions. (Luise Linsenmeier, 2017)
PRNP related signaling pathway
The PRNP protein is implicated in a variety of cellular processes including synaptic plasticity, neuronal development, and copper homeostasis. It is expressed predominantly in the central nervous system and has been linked to prion diseases, a group of fatal neurodegenerative disorders. The protein's normal function is not yet fully understood, but it is thought to play a role in cell signaling and may also act as a receptor for other molecules. Mutations in the PRNP gene can lead to inherited prion diseases, highlighting the importance of this protein in neurobiology.
PRNP related diseases
The PRNP protein, when abnormally folded, is associated with a group of fatal neurodegenerative disorders known as prion diseases. These include Creutzfeldt-Jakob disease (CJD), which can occur in sporadic, genetic, iatrogenic, and variant forms . Other prion diseases include Gerstmann-Sträussler-Scheinker syndrome and fatal familial insomnia. The PRNP gene mutation can lead to inherited forms of these diseases, and specific mutations in the PRNP gene, such as the one at codon 200, have been linked to familial CJD . Additionally, certain forms of CJD can be transmitted through medical procedures, classed as iatrogenic. Variant CJD is associated with the consumption of beef products contaminated with bovine spongiform encephalopathy (BSE). There is ongoing research into therapeutic strategies targeting the prion protein, with some studies showing potential for treatments like antibody therapies .
Bioapplications of PRNP
PRNP plays a crucial role in various bioapplications. It is primarily associated with neurodegenerative diseases such as Creutzfeldt-Jakob disease, scrapie, and bovine spongiform encephalopathy (BSE). The misfolding of PRNP into abnormal isoforms leads to the formation of amyloid plaques and neurofibrillary tangles, which disrupt cellular functions and cause neuronal death. In addition to its involvement in disease pathogenesis, PRNP has been explored for potential therapeutic applications. For instance, it has been used as a target for drug development to treat prion diseases and other neurodegenerative disorders. Moreover, PRNP-based biosensors have been developed for detecting misfolded proteins and monitoring disease progression. Overall, PRNP holds great promise for advancing our understanding of neurodegenerative diseases and developing novel diagnostic and therapeutic strategies.
Case Study
Case Study 1: Sudheer Babu Sangeetham, 2021
The formation of PrPSc, the scrapie prion, is a key occurrence in transmissible spongiform encephalopathies (TSEs), but the process of its creation from the normal prion protein (PrP) and the mechanisms involved, including any intermediates, are not completely understood. It has been reported that a variant of human PrP, specifically the G127V mutation, makes the protein resistant to TSE transmission through an unclear mechanism. Molecular dynamics simulations have hinted that this mutation might disrupt the formation of PrP dimers. In this study, researchers examine the dimerization of both 127G and 127VPrP variants, using both in vitro experiments and a mammalian cell culture model. The findings indicate that although molecular dynamics simulations can reflect the characteristics that influence dimerization outside of a cell, the inhibitory effect of G127V on PrP dimer formation is not observed within the more intricate environment of a cellular system.
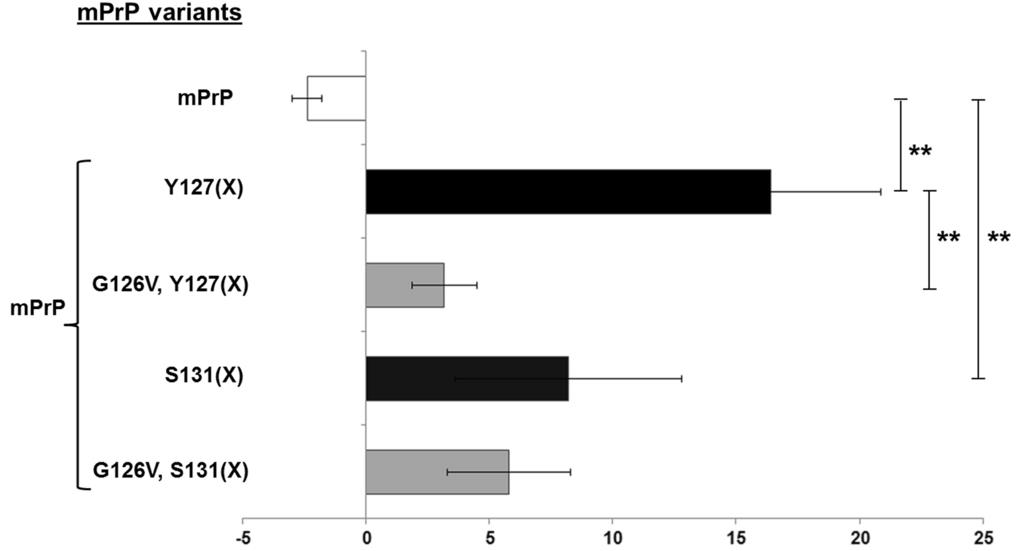
Fig1. Homodimer formation is diminished for G126V mutants of mPrPs.
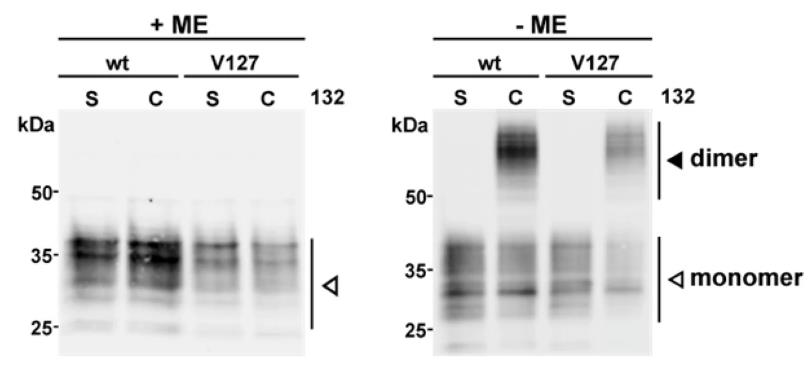
Fig2. PrP forms disulfide bond-stabilized homodimers under non-reducing conditions in Western blot analysis.
Case Study 2: Claire Lailler, 2024
Patients with EGFR-mutated NSCLC typically respond well to EGFR-targeting TKIs like osimertinib, but eventual relapse is common. One resistance mechanism, EMT, may involve the cellular prion protein PrPC, according to the hypothesis. Analyzing five lung adenocarcinoma datasets, we found PRNP gene expression, which encodes PrPC, correlates with EMT. Experiments with EGFR-mutated NSCLC cell lines showed PrPC expression is essential for a mesenchymal phenotype, possibly through an ILK-RBPJ pathway that also regulates EGFR. Higher PrPC levels were noted in EGFR-mutated tumors and with TKI resistance. Reducing PRNP made cells more sensitive to osimertinib. Plasma PrPC levels were also higher in EGFR-mutated NSCLC patients and changed with disease progression. This evidence points to PrPC as a potential driver of EMT-related TKI resistance and suggests that tracking PrPC levels could be a non-invasive way to monitor NSCLC patients' response to TKIs, opening the possibility for PrPC-targeted therapies upon TKI failure.
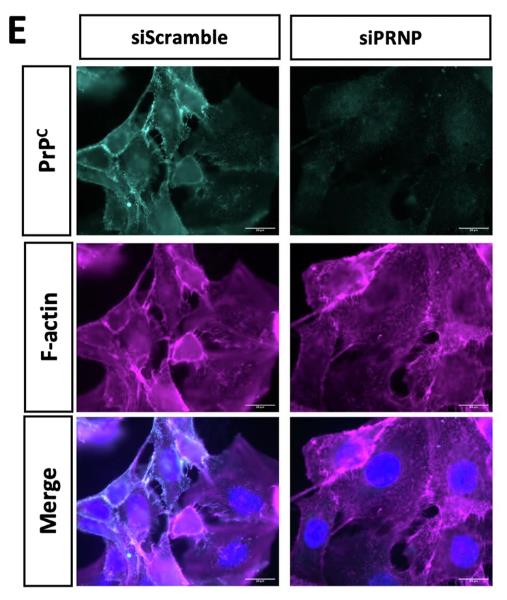
Fig3. Immunofluorescence images showing PrPC and F-actin staining in PRNP-silenced versus control H1975 cells.

Fig4. Kinetics of evolution of plasma PrPC levels in samples from EGFR-TKI patients from cohort 2 according to disease history.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (PRNP-1198H)
.
.jpg)
Fig2. SDS-PAGE (PRNP-6005H)
Involved Pathway
Prnp involved in several pathways and played different roles in them. We selected most pathways Prnp participated on our site, such as Prion diseases, which may be useful for your reference. Also, other proteins which involved in the same pathway with Prnp were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Prion diseases | PRKACG,STIP1,CASP12,MAPK1,ELK1,HSPA5,IL6,C1QA,NOTCH1,Hc |
Protein Function
Prnp has several biochemical functions, for example, ATP-dependent protein binding,chaperone binding,copper ion binding. Some of the functions are cooperated with other proteins, some of the functions could acted by Prnp itself. We selected most functions Prnp had, and list some proteins which have the same functions with Prnp. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| ATP-dependent protein binding | DDX39B,MYO5A,DNAJC5,STX1A,SAE1 |
| copper ion binding | MOXD1,SCO2,P2RX2,LOXL3,ATP7B,METTL17,HEPHL1,DCT,S100A13,SLC11A2 |
| microtubule binding | NDEL1,CLIP3,S100A9,DNM2,FTCD,DPYSL2,NDRG1,GAS2L1,SGIP1,CCDC88A |
| protein binding | JOSD1,SEPT3,CNOT8,DYNC1H1,MTA3,OTX1,CRCT1,KRTAP12-2,PDZK1,CROP |
| chaperone binding | INS1,SLC25A17,DNAJB5,DNAJB8,DNAJA3B,H2-KE2,GRPEL2,BAK1,TSC1,CDKN1B |
| tubulin binding | STMN1A,STMN1B,TTLL6,TPPP3,RABGAP1,TPR,TTLL4,NCALD,STMN4L,RITA1 |
| identical protein binding | BRAF,ACTG1,RABAC1,ALK,TPRGL,FHL2,CUBN,ECI1,UBE2G2,LEPR |
| ion channel binding | YWHAH,ID2,SUMO1,RNF207,KCNJ11,AKAP6,AP2M1,PIRT,CTNNB1,KCNIP2 |
Interacting Protein
Prnp has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with Prnp here. Most of them are supplied by our site. Hope this information will be useful for your research of Prnp.
APP
Resources
Related Services
Related Products
References
- Didonna, A; Venturini, AC; et al. Characterization of four new monoclonal antibodies against the distal N-terminal region of PrPC. PEERJ 3:-(2015).
- Salazar, E; Berriatua, E; et al. Lack of relationship between Visna/maedi infection and scrapie resistance genetic markers. SPANISH JOURNAL OF AGRICULTURAL RESEARCH 12:676-682(2014).



