IGF Binding Protein & Regulator
Related Symbol Search List
Immunology Background
Available Resources for IGF Binding Protein & Regulator Research
Creative BioMart offers extensive support for researchers studying IGFBPs and regulators, providing a range of carefully curated products, tailored services, and abundant resources.
- Our offerings include recombinant proteins, pre-coupled magnetic beads, cell and tissue lysates, and more, all designed to meet specific research needs.
- In addition to our product line, we are committed to providing a wealth of information on IGFBPs and regulators. Our resources cover essential topics such as pathways involved, protein functions, interacting proteins, related articles, and research areas, serving as a valuable reference for researchers looking to deepen their understanding of the critical role these molecules play in various physiological processes.
Our Featured Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| IGFBP1-03H | Active Recombinant Human IGFBP-1 Protein (26-259aa) | Human | HEK293 | |
| IGFBP2-29545TH | Active Recombinant Human IGFBP2 protein | Human | Insect Cell | N/A |
| IGFBP3-14107H | Recombinant Human IGFBP3, GST-tagged | Human | E.coli | GST |
| IGFBP4-759H | Active Recombinant Human IGFBP4 protein, His-tagged | Human | HEK293 | His |
| Igfbp5-1416R | Recombinant Rat Insulin-Like Growth Factor Binding Protein 5 | Rat | E.coli | N/A |
| Igfbp6-3012R | Recombinant Rat Igfbp6 protein, His/T7-tagged | Rat | E.coli | His/T7 |
| IGF2BP2-3874H | Recombinant Human IGF2BP2 protein, His&GST-tagged | Human | Insect Cells | His/GST |
| NOV-433H | Recombinant Human NOV Protein, His/GST-tagged | Human | E.coli | His/GST |
| CTGF-2605H | Recombinant Human CTGF, His-tagged | Human | HEK293 | His |
| CYR61-3656H | Recombinant Human CYR61 protein, GST-tagged | Human | E.coli | GST |
| WISP1-592H | Recombinant Human WISP1 Protein, His-tagged | Human | E.coli | His |
About IGF Binding Protein & Regulators
Insulin-like Growth Factor Binding Proteins (IGFBPs) are a group of proteins that interact with Insulin-like Growth Factors (IGFs) to regulate their bioavailability and activity. IGFBPs are essential components of the IGF system and play crucial roles in modulating IGF signaling and function. In addition to IGFBPs, there are also various regulators that influence the activity of IGFBPs. Here's an introduction to IGFBPs and their regulators:
Insulin-like Growth Factor Binding Proteins (IGFBPs)
- Structure and Function
IGFBPs are a family of proteins that bind to IGFs, including IGF-1 and IGF-2. They are structurally similar and characterized by conserved regions called IGFBP domains. In humans, there are six main IGFBPs: IGFBP-1 to IGFBP-6. IGFBPs have a high affinity for IGFs and can sequester them in the extracellular matrix, thereby regulating their availability to bind to IGF receptors.
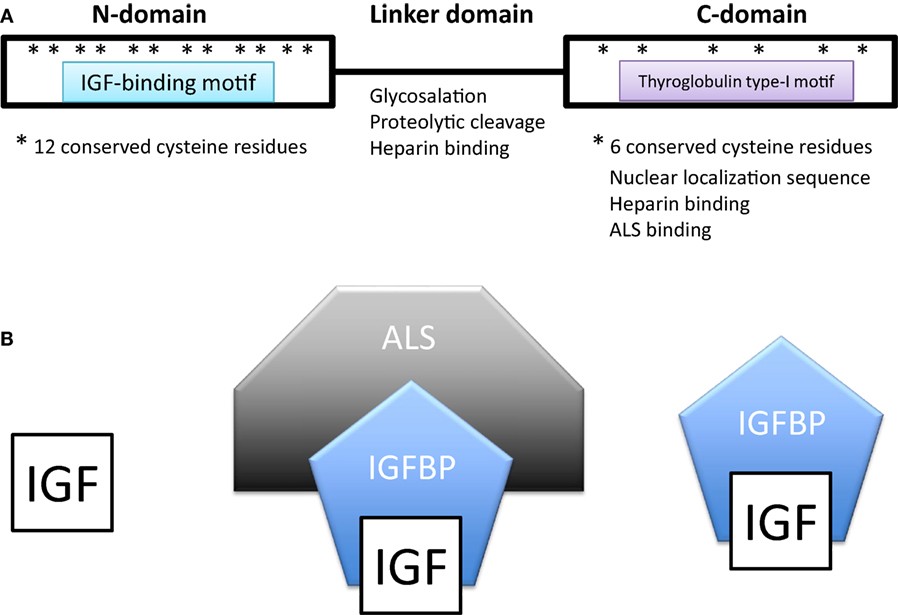 Fig.1 Domain structure of insulin-like growth factor-binding proteins (IGFBPs). (Allard JB, et al., 2018)
Fig.1 Domain structure of insulin-like growth factor-binding proteins (IGFBPs). (Allard JB, et al., 2018)
(A) Domain structure of insulin-like growth factor-binding proteins (IGFBPs). IGFBPs contain conserved N- and C-terminal domains and a variable linker domain between them. The N-domain contains an insulin-like growth factor (IGF)-binding motif and the C-domain contains a thyroglobulin type-I repeat. The N-domain usually contains 12 conserved cysteine residues and the C-domain contains 6. (B) In extracellular environments, most IGFs are bound with IGFBPs, either in a binary complex containing one IGF and one IGFBP or a ternary complex consisting of an IGF, IGFBP-3 (or less often IGFBP-5), and a glycoprotein called acid labile subunit (ALS).
- IGFBP-IGF Interactions
IGFBPs bind to IGFs with high affinity, forming stable complexes that prolong the half-life of IGFs in circulation and protect them from degradation. This binding also regulates the distribution, transport, and delivery of IGFs to target tissues. IGFBPs can enhance or inhibit the actions of IGFs depending on their interactions with other proteins and extracellular factors.
- IGFBP Proteolysis
IGFBPs can undergo proteolytic cleavage by specific proteases, resulting in the generation of smaller fragments with altered binding properties. Proteolysis of IGFBPs can modulate the availability and activity of IGFs by releasing them from the binding proteins or generating fragments that have distinct functions.
- IGFBP-Independent Actions
In addition to their role as IGF transporters, IGFBPs can also exert IGF-independent actions. Some IGFBPs can interact with cell surface receptors or other proteins, activating intracellular signaling pathways and influencing cellular processes independently of IGFs.
Regulators of IGFBPs
- IGF Proteases
Enzymes known as IGF proteases or IGFBP proteases can cleave IGFBPs, resulting in the modulation of IGF-IGFBP interactions. For example, members of the matrix metalloproteinase (MMP) family can cleave IGFBPs, thereby releasing bound IGFs and regulating their bioavailability.
- Heparin and Heparan Sulfate Proteoglycans
Heparin and heparan sulfate proteoglycans (HSPGs) are glycosaminoglycans that can bind to both IGFBPs and IGFs. They can influence the interaction between IGFBPs and IGFs, affecting their stability, distribution, and activity.
- Other Binding Proteins
Certain proteins can interact with IGFBPs and modulate their function. For example, the acid-labile subunit (ALS) can bind to IGFBPs, forming a ternary complex with IGFs. This complex enhances the stability and half-life of IGFBPs and IGFs in circulation.
The regulation of IGFBPs and their interactions with IGFs and other factors are crucial for controlling the availability and activity of IGFs in various physiological and pathological processes.
Physiological Functions of IGFBPs
IGFBPs have diverse physiological functions that extend beyond their role as transporters and regulators of IGFs. Here are some key physiological functions of IGFBPs:
- Regulation of IGF Bioavailability: IGFBPs tightly bind to IGFs, forming stable complexes that regulate the bioavailability and distribution of IGFs in the body. By sequestering IGFs, IGFBPs prolong their half-life in circulation, protect them from rapid degradation, and control their transport to target tissues. This regulation ensures the appropriate spatiotemporal availability of IGFs for their biological actions.
- Modulation of IGF Signaling: IGFBPs can modulate IGF signaling by influencing the interaction between IGFs and their cell surface receptors. Some IGFBPs, such as IGFBP-2 and IGFBP-5, have been shown to enhance IGF signaling by facilitating the binding of IGFs to their receptors. Conversely, other IGFBPs, like IGFBP-4, can inhibit IGF signaling by reducing the accessibility of IGFs to their receptors or promoting their internalization.
- Cellular Differentiation and Proliferation: IGFBPs can influence the differentiation of various cell types, including myocytes, adipocytes, osteoblasts, and chondrocytes. IGFBPs also regulate cell cycle progression and proliferation by modulating IGF signaling pathways and interacting with other signaling molecules involved in cell growth and division.
- Extracellular Matrix Interactions: Certain IGFBPs, such as IGFBP-3 and IGFBP-5, have been found to interact with components of the extracellular matrix (ECM), such as heparan sulfate proteoglycans (HSPGs) and specific ECM proteins. These interactions can modulate ECM organization, cell-ECM interactions, and cellular responses to growth factors, thereby influencing processes like tissue development, repair, and remodeling.
- Apoptosis and Cell Survival: Some IGFBPs, including IGFBP-3 and IGFBP-5, have been implicated in promoting apoptosis in certain cell types. Conversely, other IGFBPs, such as IGFBP-2 and IGFBP-4, have been shown to have anti-apoptotic effects, protecting cells from apoptosis-inducing stimuli.
- Metabolic Regulation: IGFBPs, particularly IGFBP-1 and IGFBP-2, have been associated with metabolic regulation, including glucose and lipid metabolism. IGFBP-1, for example, can regulate glucose homeostasis by inhibiting insulin signaling and promoting gluconeogenesis in the liver. The role of IGFBPs in metabolic processes highlights their involvement in energy balance, insulin sensitivity, and metabolic disorders.
The physiological functions of IGFBPs are context-dependent and can vary across different tissues, developmental stages, and pathological conditions. Their interactions with IGFs, other proteins, ECM components, and signaling pathways contribute to the regulation of cell growth, differentiation, survival, and metabolism. Understanding the complex roles of IGFBPs.
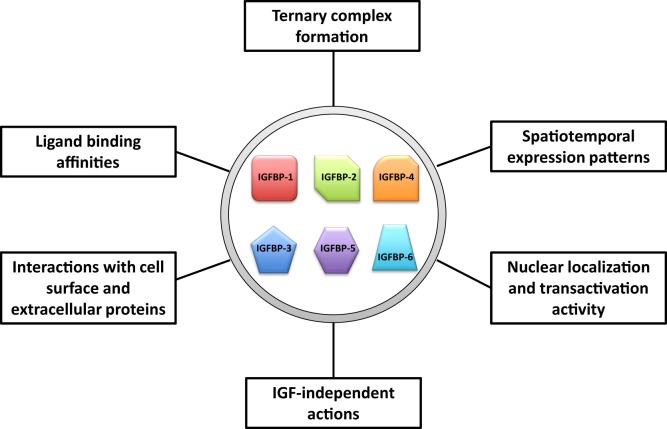 Fig.2 Major attributes of insulin-like growth factor-binding proteins (IGFBPs) that may help to give rise to the increased flexibility and versatility in their abilities to regulate insulin-like growth factor (IGF) actions. (Allard JB, et al., 2018)
Fig.2 Major attributes of insulin-like growth factor-binding proteins (IGFBPs) that may help to give rise to the increased flexibility and versatility in their abilities to regulate insulin-like growth factor (IGF) actions. (Allard JB, et al., 2018)
Common IGFBP Regulators
Several regulators can influence the expression and activity of IGFBPs, including hormones, growth factors, and cytokines. Here is a list of some regulators that can affect IGFBP expression and activity:
- Insulin-like Growth Factors (IGFs): IGFs themselves can regulate the expression and activity of IGFBPs. IGFs can induce the synthesis of certain IGFBPs, such as IGFBP-1 and IGFBP-3, in a feedback loop to modulate their own availability and activity. Additionally, IGFs can influence the proteolytic processing and secretion of IGFBPs.
- Growth Hormone (GH): GH plays a role in regulating IGFBP expression. GH can increase the synthesis and secretion of IGFBP-3 and IGFBP-5 in the liver. It also influences the levels of other IGFBPs, including IGFBP-1, IGFBP-2, and IGFBP-4, in various tissues.
- Thyroid Hormones: Thyroid hormones, such as triiodothyronine (T3), can modulate IGFBP expression. T3 has been shown to increase the levels of IGFBP-2 and IGFBP-3 in certain tissues. The regulation of IGFBPs by thyroid hormones contributes to the coordination of growth and metabolism.
- Sex Steroids: Sex steroids, including estrogen and testosterone, can influence IGFBP expression. Estrogen has been reported to increase the levels of IGFBP-3, while testosterone can decrease IGFBP-1 expression. These hormonal regulations contribute to gender-specific differences in IGFBP profiles.
- Transforming Growth Factor-beta (TGF-β): TGF-β is a cytokine that can affect IGFBP expression. TGF-β can upregulate the expression of IGFBP-3 and IGFBP-5 in certain cell types. The interaction between TGF-β and IGFBPs plays a role in tissue development, wound healing, and fibrosis.
- Interleukins (ILs): Certain interleukins, such as IL-1 and IL-6, can regulate IGFBP expression. For example, IL-1β can induce the expression of IGFBP-3, while IL-6 can modulate the levels of IGFBP-1 and IGFBP-3. The influence of ILs on IGFBPs is associated with inflammatory processes and immune responses.
- Insulin and Glucose: Insulin and glucose levels can affect IGFBP expression and activity. Insulin can decrease the levels of IGFBP-1, while glucose can increase IGFBP-1 expression. Insulin and glucose also influence the proteolytic processing of IGFBPs, affecting their stability and availability.
- Hypoxia-Inducible Factors (HIFs): HIFs are transcription factors that respond to hypoxic conditions. HIFs can regulate IGFBP expression under hypoxia. For instance, HIF-1α can upregulate the expression of IGFBP-1 and IGFBP-3, contributing to adaptive responses to low oxygen levels.
These are just a few examples of the regulators that can influence the expression and activity of IGFBPs. The intricate interplay between various hormones, growth factors, cytokines, and metabolic factors contributes to the dynamic control of IGFBP levels, impacting the availability and function of Insulin-like Growth Factors and influencing diverse physiological processes.
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
Related References
- Allard JB, Duan C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front Endocrinol (Lausanne). 2018;9:117.
- Clemmons DR. Role of IGF Binding Proteins in Regulating Metabolism. Trends Endocrinol Metab. 2016;27(6):375-391.
