Recombinant Full Length Human Parainfluenza 3 Virus Hemagglutinin-Neuraminidase(Hn) Protein, His-Tagged
| Cat.No. : | RFL8691HF |
| Product Overview : | Recombinant Full Length Human parainfluenza 3 virus Hemagglutinin-neuraminidase(HN) Protein (P12564) (1-572aa), fused to N-terminal His tag, was expressed in E. coli. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | HPIV3 |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | Full Length (1-572) |
| Form : | Lyophilized powder |
| AA Sequence : | MEYWKHTNHGKDAGNELETSMATHGNKLTNKIIYILWTIILVLLSIVFIIVLINSIKSEK AHESLLRDINNEFMEITGKIQMASDNTNDLIQSGVNTRLLTIQSHVQNYIPISLTQQMSD LRKFISEITIRNDNQEVLPQRITHDVGIKPLNPDDFWRCTSGLPSLMKTPKIRLMPGPGL LAMPTTDDGCIRTPSLVINDLIYAYTSNLITRGCQDIGKSYQVLQIGIITVNSDLVPDLN PRISHTFNINDNRKSCSLALLNTDVYQLCSTPKVDERSDYASSGIEDIVLDIVNYDGSIS TTRFKNNNISFDQPYAALYPSVGPGIYYKGKIIFLGYGGLEHPINENVICNTTGCPGKTQ RDCNQASHSPWFSDRRMVNSIIVVDKGLNSTPKLKVWTISMRQNYWGSEGRLLLLGNKIY IYTRSTSWHSKLQLGIIDITDYSDIRIKWTWHNVLSRPGNNECPWGHSCPDGCITGVYTD AYPLNPTGSIVSSVILDSQKSRVNPVITYSTATERVNELAIRNRTLSAGYTTTSCITHYN KGYCFHIVEINHKSLNTFQPMLFKTEIPKSCS |
| Purity : | Greater than 90% as determined by SDS-PAGE. |
| Applications : | SDS-PAGE |
| Notes : | Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week. |
| Storage : | Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles. |
| Storage Buffer : | Tris/PBS-based buffer, 6% Trehalose, pH 8.0 |
| Reconstitution : | We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference. |
| Gene Name | HN |
| Synonyms | HN; Hemagglutinin-neuraminidase |
| UniProt ID | P12564 |
Case 1: Wu X, et al. PLoS Pathog. 2023
Many viruses initiate infection by binding to sialoglycan receptors at the cell surface. Binding to such receptors comes at a cost, however, as the sheer abundance of sialoglycans e.g. in mucus, may immobilize virions to non-functional decoy receptors. As a solution, sialoglycan-binding as well as sialoglycan-cleavage activities are often present in these viruses, which for paramyxoviruses are combined in the hemagglutinin-neuraminidase (HN) protein. The dynamic interactions of sialoglycan-binding paramyxoviruses with their receptors are thought to be key determinants of species tropism, replication and pathogenesis. Here researchers used biolayer interferometry to perform kinetic analyses of receptor interactions of animal and human paramyxoviruses (Newcastle disease virus, Sendai virus, and human parainfluenza virus 3). They show that these viruses display strikingly different receptor interaction dynamics, which correlated with their receptor-binding and -cleavage activities and the presence of a second sialic acid binding site. Virion binding was followed by sialidase-driven release, during which virions cleaved sialoglycans until a virus-specific density was reached, which was largely independent of virion concentration. Here researchers propose that paramyxoviruses display sialidase-driven virion motility on a receptor-coated surface, until a threshold receptor density is reached at which virions start to dissociate.
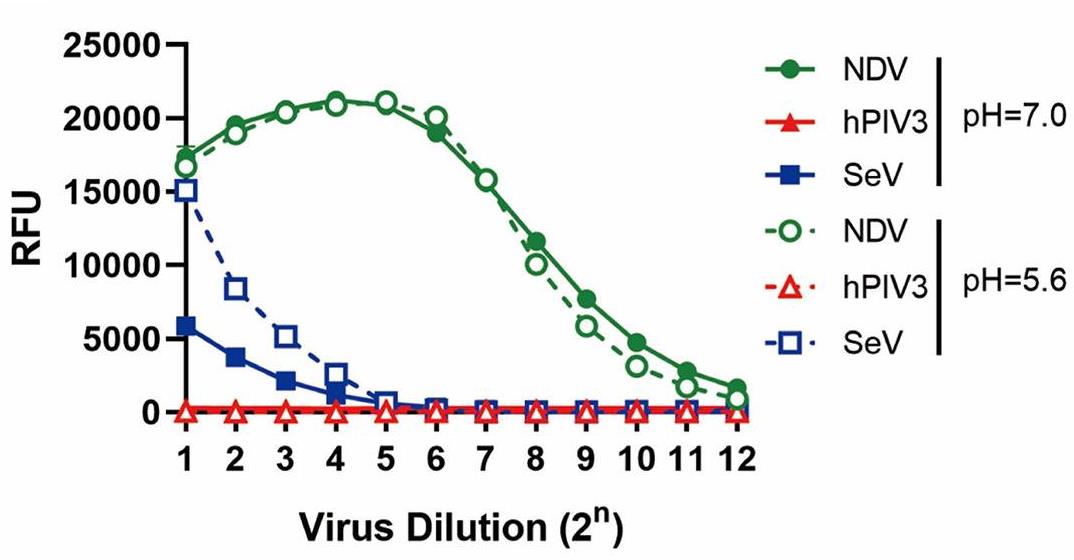
Fig1. Sialidase activity assay.
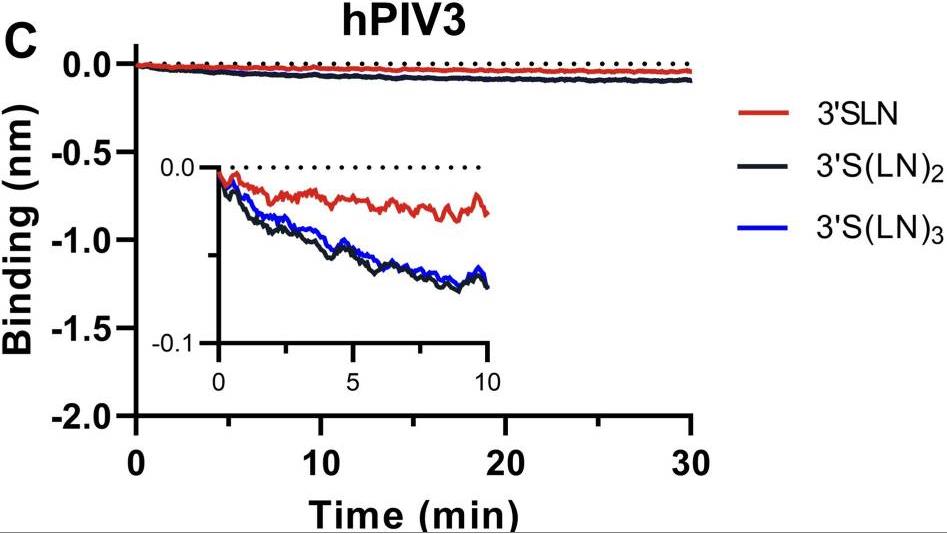
Fig2. Analysis of the ability of hPIV3 binding ability to 3'SLN, 3'S(LN)2, or 3'S(LN)3.
Case 2: Marcink TC, et al. Sci Adv. 2023
Paramyxoviruses-including important pathogens like parainfluenza, measles, and Nipah viruses-use a receptor binding protein [hemagglutinin-neuraminidase (HN) for parainfluenza] and a fusion protein (F), acting in a complex, to enter cells. Researchers use cryo-electron tomography to visualize the fusion complex of human parainfluenza virus 3 (HN/F) on the surface of authentic clinical viruses at a subnanometer resolution sufficient to answer mechanistic questions. An HN loop inserts in a pocket on F, showing how the fusion complex remains in a ready but quiescent state until activation. The globular HN heads are rotated with respect to each other: one downward to contact F, and the other upward to grapple cellular receptors, demonstrating how HN/F performs distinct steps before F activation.
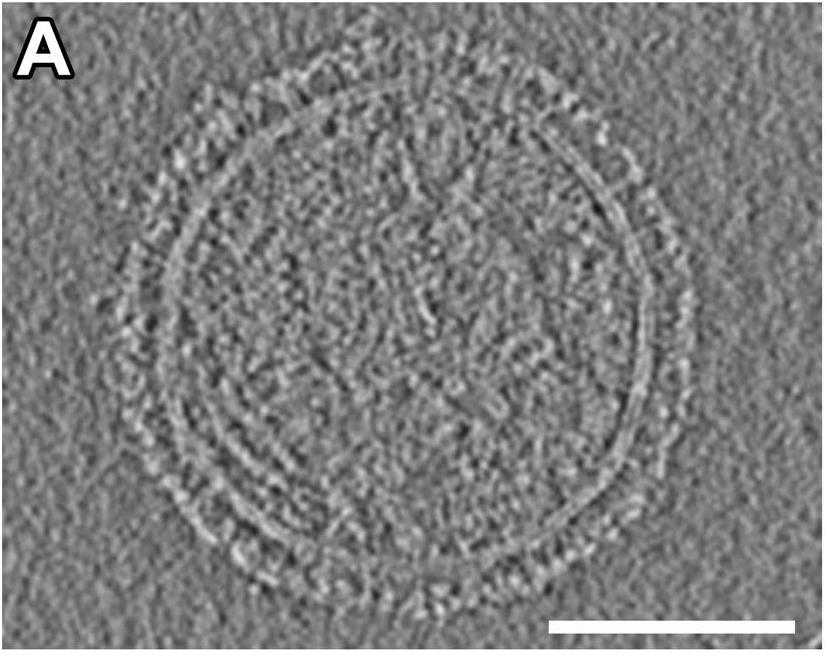
Fig1. Representative central slice from a tomogram of an HPIV3 viral particle.
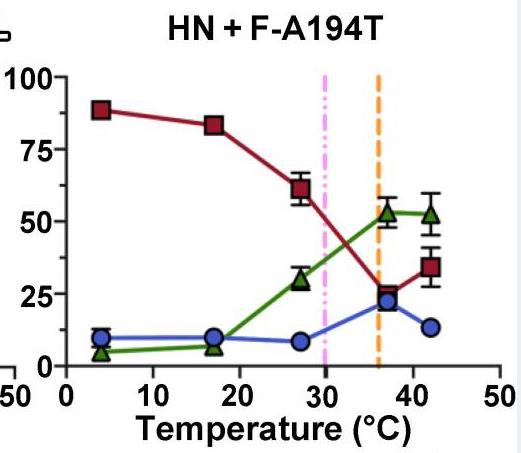
Fig2. Activation of F by HN was assessed at a range of temperatures with F-A194T.
Parainfluenza 3 Virus Hemagglutinin-Neuraminidase (HN) protein, as a viral structural protein, has been used in the field of disease-related research and the development of therapeutic reagents. There are mainly the following:
Basic research: The HN protein is also an important model protein in virology and immunology research, helping to understand how viruses interact with host cells and how to develop effective antiviral strategies.
Vaccine development: As a key surface glycoprotein of Newcastle Disease virus (NDV), HN protein can induce the production of neutralizing antibodies, which makes HN protein a potential target for vaccine development. By expressing HN protein in rice endosperm, researchers have successfully developed a semi-quantitative rapid immunochromatographic assay for the detection of NDV antibodies, which helps to evaluate the immunological efficacy of the vaccine.
Biopharmaceutical: The expression and purification of HN protein provides a new production platform for the biopharmaceutical industry. Compared with traditional bacterial and yeast fermentation or mammalian cell production, plant molecular agriculture has the advantages of low production cost, easy storage and high yield.
Disease treatment: Because NDVS have the ability to effectively infect and induce apoptosis in tumor cells, HN proteins have potential applications in the study of NDVS as oncolytic viruses, helping to develop new cancer treatment strategies.
Development of diagnostic reagents: Immunochromatographic test strips developed using HN protein can be used for rapid, simple, sensitive and specific NDV antibody detection, providing a powerful tool for epidemic surveillance and vaccine effectiveness evaluation.
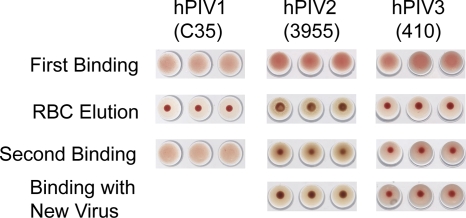
Fig1. N action on chicken (hPIV1 and -2) or turkey (hPIV3) red blood cells. (Mary M Tappert, 2011)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All HN Products
Required fields are marked with *
My Review for All HN Products
Required fields are marked with *
Inquiry Basket


