Active Recombinant Protease
| Cat.No. : | Protease-92 |
| Product Overview : | Recombinant Protease is a fusion protein of glutathione S-transferase (GST) and human rhinovirus (HRV) type 14 3C protease. The protease specifically recognizes a subset of sequences which include the core amino acid sequence Leu-Phe-Gln/Gly-Pro cleaving between the Gln and Gly residues. Substrate recognition and cleavage are likely to be dependent not only upon primary structural signals, but also upon the secondary and tertiary structures of the fusion protein as well. The Recombinant Protease is purified by proprietary chromatographic techniques. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Source : | E.coli |
| Tag : | GST |
| Physical Appearance : | Sterile filtered colorless solution. |
| Cleavage Buffer : | 50 mM Tris-HCl, pH-7.0 (at 25°C), 150 mM NaCl, 1 mM EDTA, 1mM dithiothreitol. Chill to 5°C prior to use. |
| Cleavage Conditions : | For Cleavage of a Fusion Protein: During cleavage reactions, it is recommended that samples be removed at various time points and analyzed by SDS-PAGE to estimate the yield, purity, and extent of digestion. The amount of PreScission Protease, temperature and length of incubation required for complete digestion of a given GST fusion partner may vary depending on the fusion partner. Optimal conditions for each fusion should be determined in pilot experiments. Digestion may be improved by adding TritonTM X-100, TweenTM 20, NonidetTM, or NP40 to a concentration of 0.01%. Concentrations of these detergents up to 1% do not inhibit PreScission Protease. |
| Unit Defenition : | One unit will cleave ≥90% of 100 µg of a test GST-fusion protein in Cleavage Buffer (50mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, pH 7.0 at 25°°C) at 5°C for 16 hours. |
| Stability : | Recombinant Protease although stable at 14°C for 1 week, should be stored desiccated below -18°C. Please prevent freeze thaw cycles. |
| Synonyms | Protease; Peptide Hydrolases; EC 3.4-; Glutamic acid proteases. |
| ◆ Recombinant Proteins | ||
| Protease-308P | Active Recombinant TEV Protease Protein (231 aa), N-His-tagged | +Inquiry |
| Protease-92 | Active Recombinant Protease | +Inquiry |
| Protease-01 | Active Recombinant Human Protease Protein, 6×His tagged | +Inquiry |
| Protease-23T | Active Recombinant TEV Protease, His-tagged | +Inquiry |
| Protease-75T | Active Recombinant TEV Protease (AA 5-236), N-GFP and C-His-Tagged | +Inquiry |
| ◆ Native Proteins | ||
| Protease-20S | Active Native Streptomyces griseus Protease | +Inquiry |
Case 1: Afrand M, et al. Int J Biol Macromol. 2024
The serine protease was produced recombinantly in E. coli and attached to an amino graphene-chitosan nanohybrid. Analysis via FTIR, XRD, DLS, and FE-SEM confirmed the enzyme's successful immobilization, which boosted its temperature optimum to 70°C and enhanced thermal and pH stability. After 30 days, the immobilized enzyme maintained 83% activity versus 56% for the free enzyme. This method improved kinetic and thermodynamic properties, marking a significant step in enzyme stabilization through synthetic biology and nanomaterial science.

Fig1. Immobilized rSAPV on AG/CS nanohybrid.
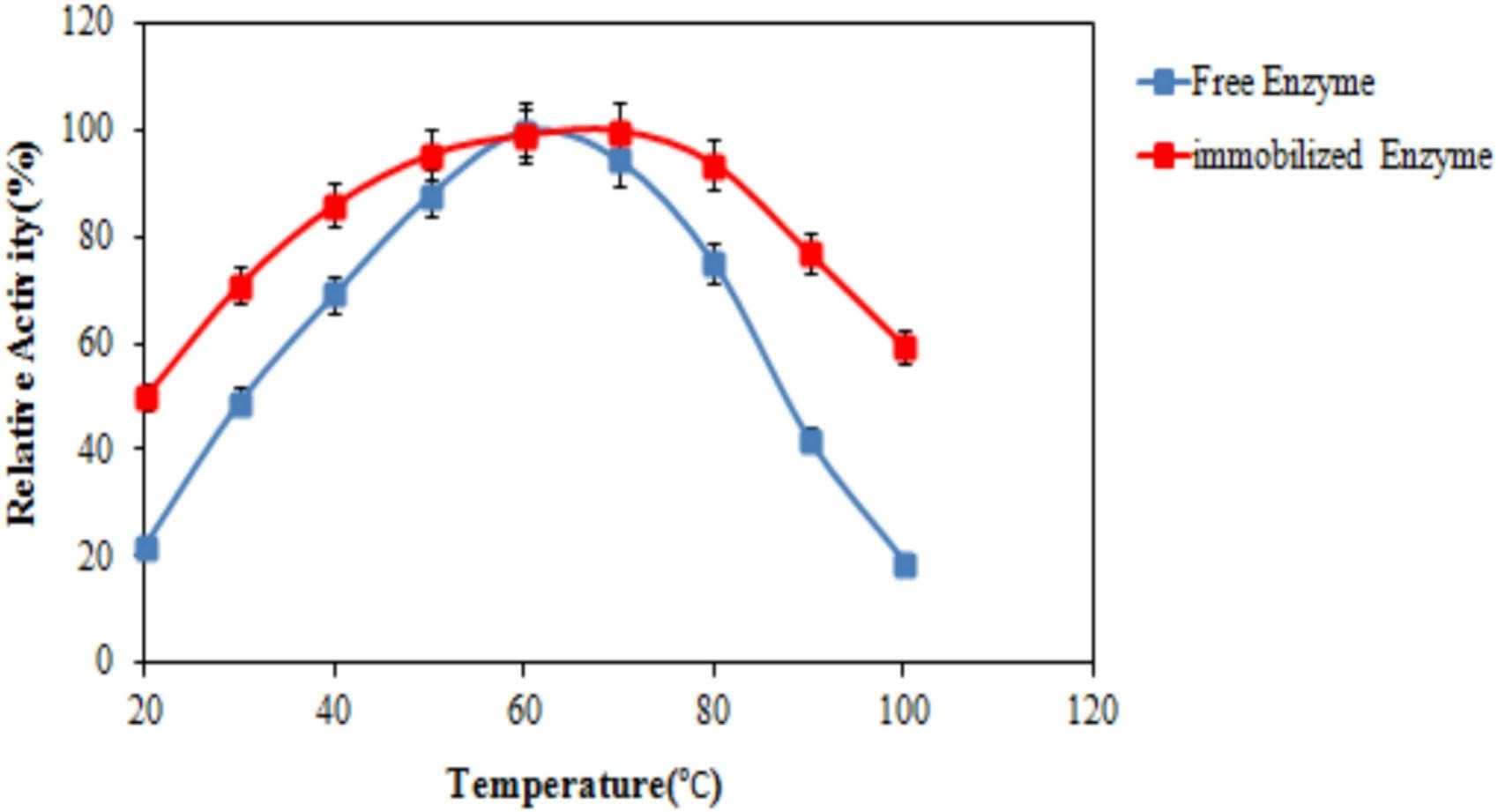
Fig2. Impact of temperature on the activity of rSAPV enzyme.
Case 2: Mushtaq A, et al. Biology (Basel). 2024
The thermophilic protease gene (nprB) from Streptomyces thermovulgaris was successfully cloned and expressed in E. coli, yielding a soluble enzyme with a molecular mass of approximately 70 kDa. The optimal conditions for expression were 1 mM IPTG and 37 °C for 4 hours. The purified enzyme showed high activity at pH 7.2 and 70 °C, and retained 80% of its activity across pH 6-8 after 4 hours. It was identified as a metalloprotease, with its activity completely inhibited by EDTA and enhanced by certain metal ions. The protease was also stable in the presence of organic solvents and surfactants, indicating its potential for use in industries such as detergents, laundry, leather, and pharmaceuticals.
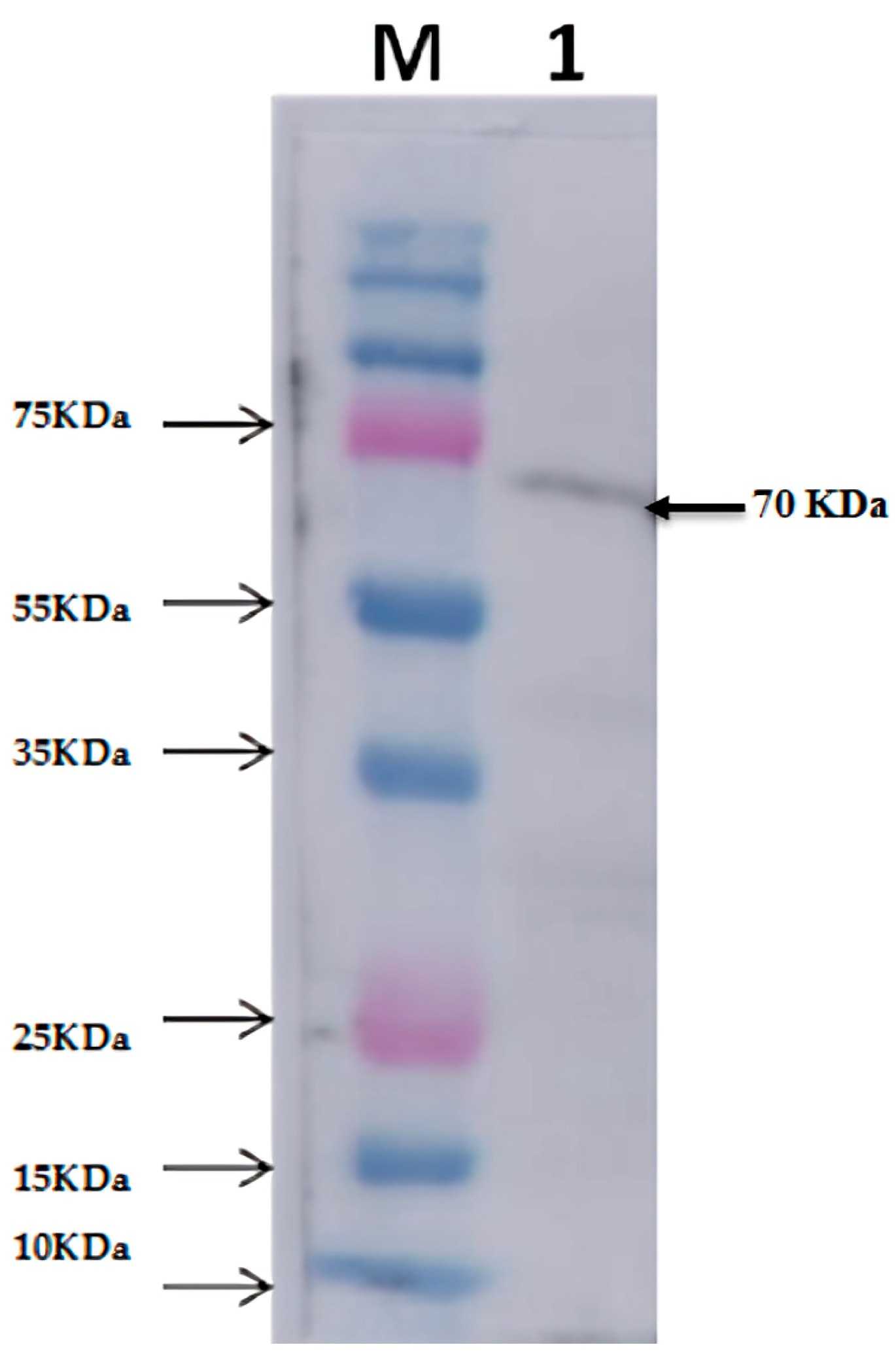
Fig1. Western blot analysis for protease expression extracted from the E. coli BL21 DE3 harboring recombinant (nprB).
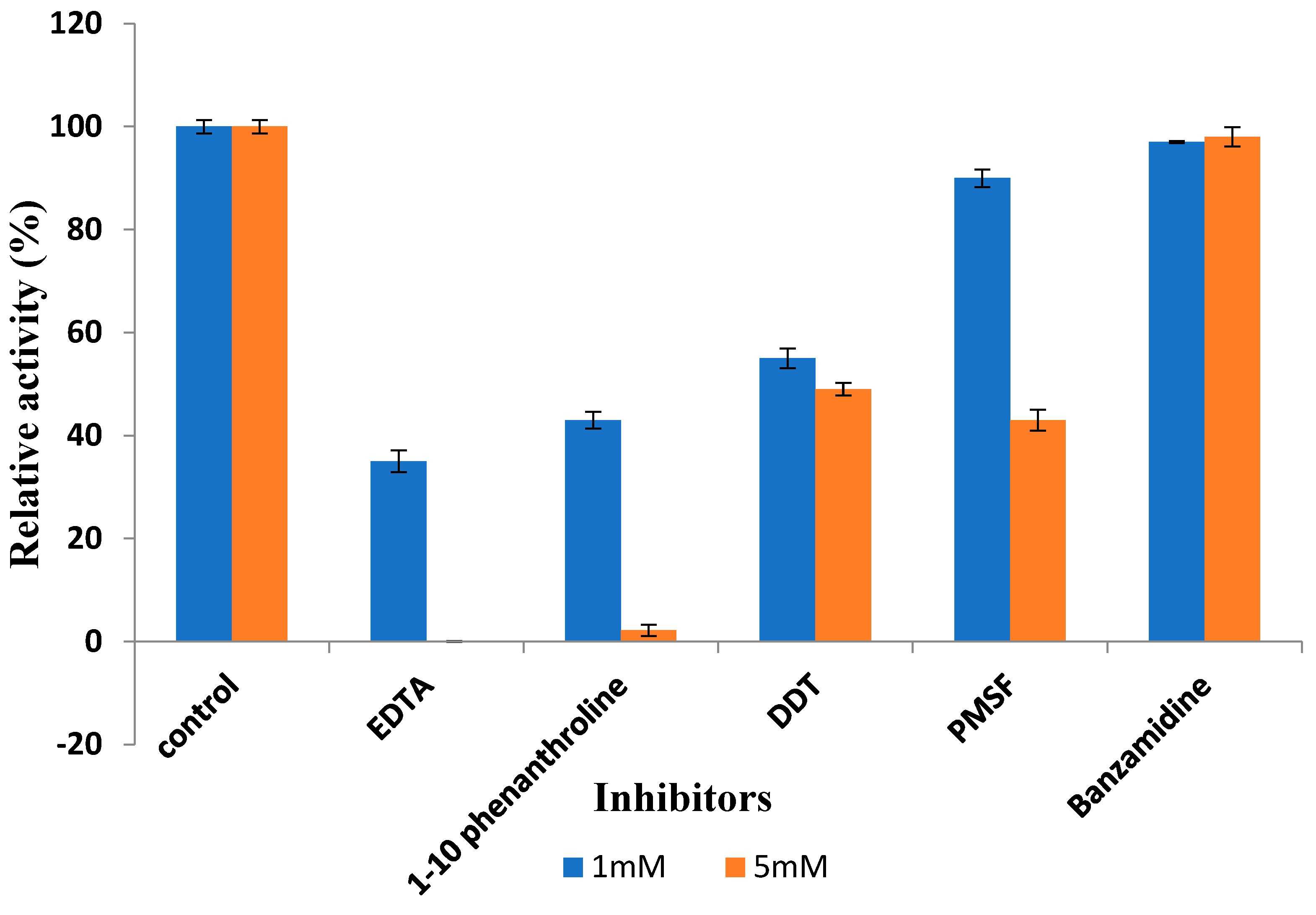
Fig2. Effect of different inhibitors on protease activity at 1 mM and 5 mM concentrations.
Active Recombinant Protease is a protein with catalytic activity expressed in host cells through genetic engineering techniques. They have a wide range of applications in several fields:
Biomedical research: In cell biology, molecular biology, and biochemistry research, recombinant proteases are used as research tools to help scientists explore the function and interactions of proteins.
Drug development: Recombinant proteases are used in the process of drug screening and development to identify and validate targets for new drugs, as well as in the study of drug mechanisms of action.
Diagnostic reagents: In clinical diagnosis, recombinant protease is used as a key component in the development of rapid and sensitive assays such as enzyme-linked immunosorbent assay (ELISA) and other biochemical assays.
Food industry: In food processing, specific proteases are used to improve the texture, flavor and nutritional value of foods, for example in cheese manufacturing and baking processes.
Biotechnology: In the field of biotechnology, recombinant proteases are used in the production of biofuels, bioplastics and other bio-based materials.
Agriculture: In agriculture, proteases are used as feed additives to improve the nutritional value of feed and the production performance of animals.
Cosmetics industry: In cosmetics manufacturing, proteases are used to develop anti-aging products and other skin care products because of their ability to promote skin cell renewal and improve skin condition.
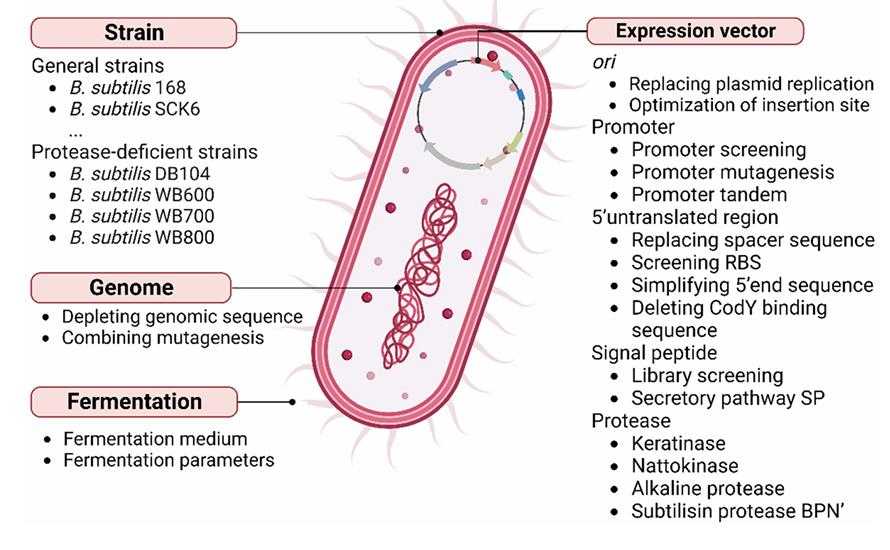
Fig1. Genetic strategies for improving recombinant protease secretion in Bacillus. Subtilis. (Xiufang Liu, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All Protease Products
Required fields are marked with *
My Review for All Protease Products
Required fields are marked with *
Inquiry Basket


