TXN
-
Official Full Name
thioredoxin -
Overview
The protein encoded by this gene acts as a homodimer and is involved in many redox reactions. The encoded protein is active in the reversible S-nitrosylation of cysteines in certain proteins, which is part of the response to intracellular nitric oxide. This protein is found in the cytoplasm. Two transcript variants encoding different isoforms have been found for this gene. -
Synonyms
TXN;thioredoxin;TRX;ADF;SASP;TXN delta 3;ATL-derived factor;thioredoxin delta 3;surface-associated sulphydryl protein;TRDX;TRX1;MGC61975;DKFZp686B1993
Recombinant Proteins
- Human
- Yeast
- Rhesus macaque
- Zebrafish
- Chicken
- E.coli
- Human
- Mammalian Cells
- HEK293
- Non
- GST
- His
- Strep
- DDK
- Myc
- Avi
- Fc
- Flag
Background
What is TXN protein?
TXN (thioredoxin) gene is a protein coding gene which situated on the long arm of chromosome 9 at locus 9q31. TXN is a multifunctional REDOX enzyme involved in many REDOX reactions in cells. It protects cells from oxidative stress by maintaining the reduced state of proteins through the reversible oxidation of the disulfide bond, its active center. TXN can catalyze disulfide bond and disulfide bond exchange reactions and participate in many REDOX processes in cells. TXN is involved in cell signaling and transcriptional regulation by regulating the activity of transcription factors, such as activating AP-1 (FOS/JUN) DNA binding activity. TXN system is an important part of cell homeostasis, bioenergy, detoxification and cell signal transduction network. TXN protein is consisted of 105 amino acids and its molecular mass is approximately 11.7 kDa.
What is the function of TXN protein?
TXN participates in a variety of REDOX reactions, interacting with sulfhydryl groups (-SH) on proteins through the disulfide bond (-S-S-) in its active center to maintain the reduced state of proteins. TXN helps clear reactive oxygen species (ROS) from within cells and protects cells from damage caused by oxidative stress. TXN is involved in the regulation of intracellular signaling pathways, affecting cell growth, differentiation and death. TXN is involved in intracellular nitric oxide (NO) signaling and regulates protein function through S-nitrosylation. TXN can activate transcription factors, such as activating the DNA-binding activity of AP-1 (composed of FOS and JUN proteins), thereby affecting gene expression.
TXN Related Signaling Pathway
TXN interacts with the NF-κB signaling pathway and is involved in regulating cellular inflammatory and immune responses. TXN interacted with Thioredoxin Interacting Protein TXNIP (TXNIP), which inhibited the antioxidant functions of TXN and affected the intracellular REDOX balance. TXN is involved in cell proliferation and cell cycle regulation by influencing DNA binding activity such as AP-1 (composed of FOS and JUN proteins). TXN is involved in regulating cellular metabolic pathways, including biological processes such as electron transport, transcription, transcriptional regulation, and substance transport.
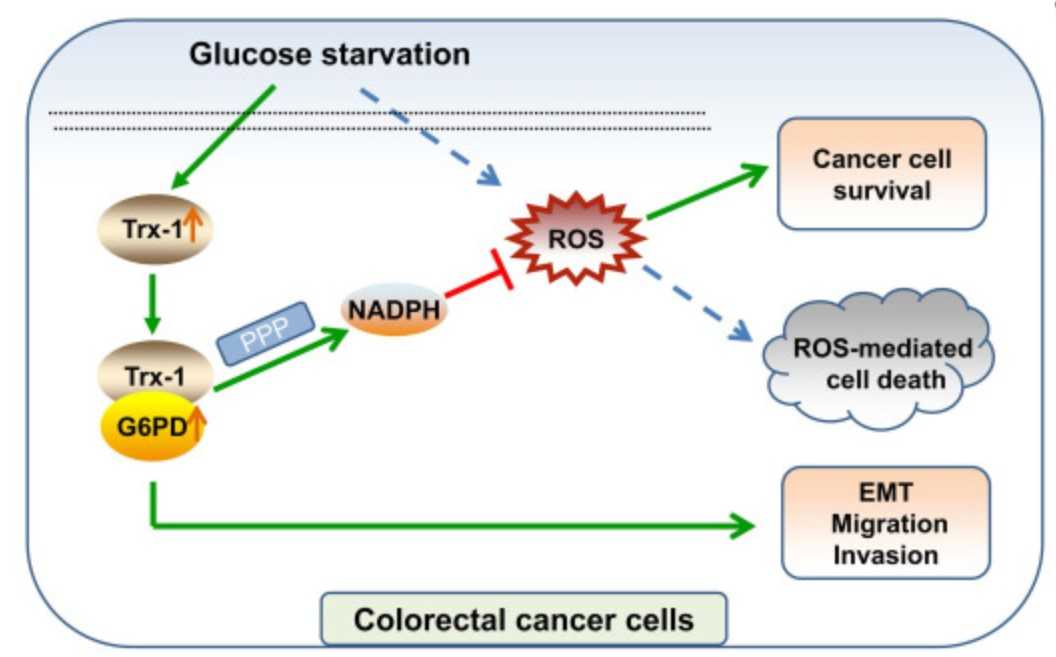
Fig1. A schematic diagram showing that Trx-1 supports colorectal cancer cell survival and promotes migration and invasion under glucose deprivation. (Fengying Lu, 2022)
TXN Related Diseases
TXN plays a role in many types of cancer, including promoting tumor cell proliferation, metastasis, and drug resistance. The TXN system is associated with diabetes and may be involved in the development of diabetes by influencing oxidative stress and inflammatory responses. TXN may play a biological role in urinary calculi, although the specific mechanism needs to be further studied. TXN interacting proteins (TXNIP) have been implicated in the development and progression of lung disease, possibly by influencing REDOX status and inflammatory response. In addition, it is also associated with cardiovascular diseases, nervous system diseases, inflammatory diseases and so on.
Bioapplications of TXN
As a protein, TXN has a variety of drug targets. The drugs targeting TXN are mainly divided into drugs that inhibit TXN function and drugs that stimulate TXN function. Recombinant TXN has been studied for the treatment of a variety of diseases, including tumors, liver disease, and heart disease. For example, in tumor therapy, recombinant TXN can be used as a carrier of tumor vaccine for tumor immunotherapy. In some studies, recombinant TXN has been used to prepare therapeutic proteins with biological activity, such as in experimental studies for the treatment of ulcerative colitis.
Case Study
Case Study 1: Fengying Lu, 2022
Overcoming energy stress is a critical step for cells in solid tumors. Under this stress microenvironment, cancer cells significantly alter their energy metabolism to maintain cell survival and even metastasis. Previous studies have shown that thioredoxin-1 (Trx-1) expression is increased in colorectal cancer (CRC) and promotes cell proliferation. However, the exact role and mechanism of how Trx-1 is involved in energy stress are still unknown. Here, the researchers observed that glucose deprivation of CRC cells led to cell death and promoted the migration and invasion, accompanied by upregulation of Trx-1. Increased Trx-1 supported CRC cell survival under glucose deprivation. Whereas knockdown of Trx-1 sensitized CRC cells to glucose deprivation-induced cell death and reversed glucose deprivation-induced migration, invasion, and epithelial-mesenchymal transition (EMT). Furthermore, they identified glucose-6-phosphate dehydrogenase (G6PD) interacting with Trx-1 by HuPortTM human protein chip, co-IP and co-localization. Trx-1 promoted G6PD protein expression and activity under glucose deprivation, thereby increasing nicotinamide adenine dinucleotide phosphate (NADPH) generation. Inhibition of Trx-1 and G6PD, together with inhibition of glycolysis using 2-deoxy-D-glucose (2DG), resulted in significant anti-tumor effects in CRC xenografts in vivo.
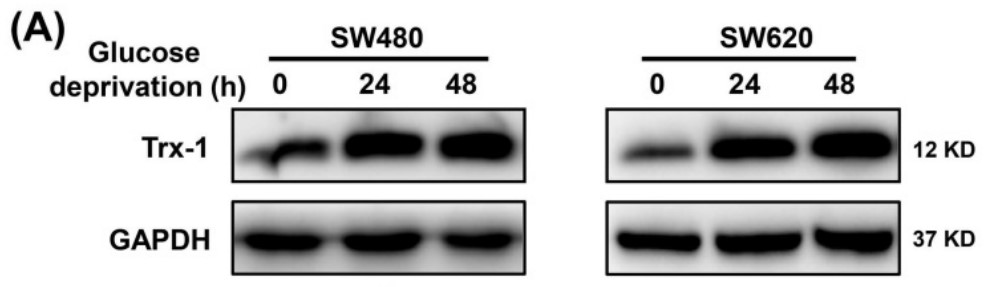
Fig1. Protein expression of Trx-1 was detected by Western blot analysis.
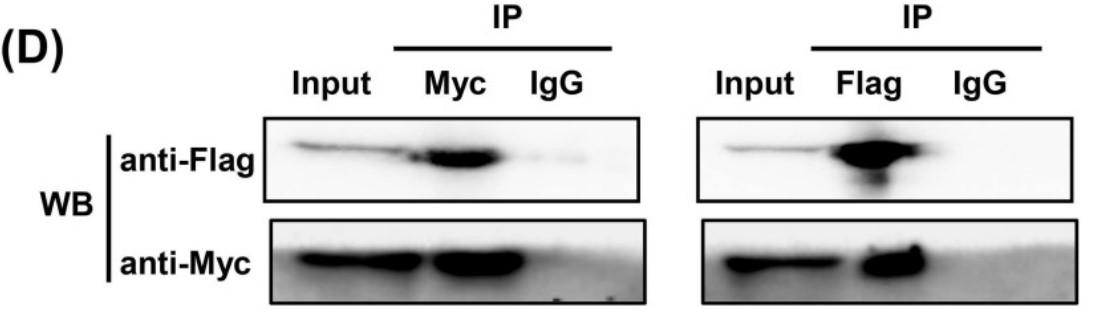
Fig2. The interaction between Trx-1 and G6PD was confirmed by co-IP assays.
Case Study 2: Ying Yang, 2020
To improve the clinical outcome of adoptive NK cell therapy in patients with solid tumors, NK cells need to persist within the tumor microenvironment (TME) in which the abundance of ROS could dampen antitumor immune responses. In the present study, the researchers demonstrated that IL-15-primed NK cells acquired resistance against oxidative stress through the thioredoxin system activated by mTOR. Mechanistically, the activation of thioredoxin showed dependence on localization of thioredoxin-interacting protein. They show that NK cells residing in the tumor core expressed higher thiol densities that could aid in protecting other lymphocytes against ROS within the TME. Furthermore, the prognostic value of IL15 and the NK cell gene signature in tumors may be influenced by tobacco smoking history in patients with non-small-cell lung cancer (NSCLC).
 in NK cells.jpg)
Fig3. Representative histogram and relative MFI of thioredoxin (Trx1) in NK cells.
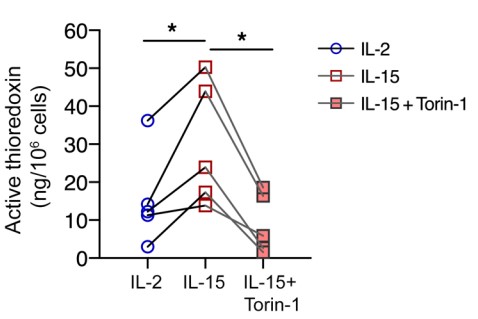
Fig4. Concentration of active thioredoxin protein in NK cells.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (TXN-155H)
.
.jpg)
Fig2. SDS-PAGE (TXN-2132H)
Involved Pathway
TXN involved in several pathways and played different roles in them. We selected most pathways TXN participated on our site, such as Cellular Senescence,Cellular responses to stress,Detoxification of Reactive Oxygen Species, which may be useful for your reference. Also, other proteins which involved in the same pathway with TXN were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Immune System | CLEC5A,TRIM8,CASPB,WIPF3,TRIM10,TREML1,DEFB121,GBP2,LAIR2,DEFA6 |
| Detoxification of Reactive Oxygen Species | PRDX3,PRDX2,TXNRD2,Txn1,TXN2 |
| Cellular responses to stress | RNF2,CDK6,EEF1A1,PRDX3,RRAGA,SUZ12A,PHC3,UBN1,HTATIP,HIST3H2BB |
| Inflammasomes | TXNIPB,BTR02,BTR12,BTR16,BTR18,SUGT1,BTR30,BTR22,PANX1A,BTR06 |
| Generic Transcription Pathway | LRPPRC,ZNF223,ZNF480,ZNF530,COX6A1,COX6B1,ZNF544,MED31,RORAA,TRIM33 |
| Gene Expression | HENMT1,QTRT1,PEAK1,ZNF215,POLR3F,HNRPL,XPOT,ZNF484,SKILB,ZNF334 |
| Cellular Senescence | CBX8B,CDK6,AGO3,EED,KDM6B,CDK4,ASF1A,E2F3,HIST1H1C,PHC3 |
| Folic Acid Network | SEPW1,FMN1 |
Protein Function
TXN has several biochemical functions, for example, disulfide oxidoreductase activity,oxidoreductase activity, acting on a sulfur group of donors, disulfide as acceptor,protein disulfide oxidoreductase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by TXN itself. We selected most functions TXN had, and list some proteins which have the same functions with TXN. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| protein disulfide oxidoreductase activity | CCS,ERO1LB,GRX2,SH3BGRL3,GFER,CHCHD4,ERO1L,ENOX2,TXNRD1,TXN2 |
| peptide disulfide oxidoreductase activity | Txn1,TXNDC12 |
| poly(A) RNA binding | MTAP4,HIST1H4E,GTF2E2,PIGC,ZC3H10,EIF3G,WDR46,CCDC9,RPL5,HNRNPH3 |
| disulfide oxidoreductase activity | GRX2,PDIA3,PDIA2,TMX1,DNAJC10,COIL,TXNL1 |
| protein binding | TEAD4,SYNJ2,LOXL2,AAGAB,ERLIN2,NATD1,CHD2,SPAG8,CCDC117,MED30 |
| oxidoreductase activity, acting on a sulfur group of donors, disulfide as acceptor | DNAJC10,ERO1LB,TXNL1,TXN2,TXNDC8,MSRB1A,MSRB1B,ERO1L |
Interacting Protein
TXN has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with TXN here. Most of them are supplied by our site. Hope this information will be useful for your research of TXN.
EGFR;COPS5;slrP;MYD88
Resources
Related Services
Related Products
References
- Singh, P; Feld, RI; et al. Sensitization, pathologic, and imaging findings comparing symptomatic and quiescent failed renal allografts. CLINICAL TRANSPLANTATION 28:1424-1432(2014).
- Negroni, L; Taouji, S; et al. Integrative Quantitative Proteomics Unveils Proteostasis Imbalance in Human Hepatocellular Carcinoma Developed on Nonfibrotic Livers. MOLECULAR & CELLULAR PROTEOMICS 13:3473-3483(2014).



