MOG
-
Official Full Name
myelin oligodendrocyte glycoprotein -
Overview
The product of this gene is a membrane protein expressed on the oligodendrocyte cell surface and the outermost surface;of myelin sheaths. Due to this localization, it is a primary target antigen involved in immune-mediated demyelination.;This protein may be involved in completion and maintenance of the myelin sheath and in cell-cell communication.;Alternatively spliced transcript variants encoding different isoforms have been identified. -
Synonyms
MOG;myelin oligodendrocyte glycoprotein;myelin-oligodendrocyte glycoprotein;MOG Ig-AluB;MOG alpha-5;MOGIG2;NRCLP7;MGC26137
Recombinant Proteins
- Human
- Rat
- Mouse
- Cattle
- Cynomolgus
- Mouse/Rat
- Macaca fascicularis
- Callithrix jacchus (White-tufted-ear marmoset)
- Pongo Abelii
- Bovine
- E.coli
- HEK293
- Synthetic
- Human Cells
- Mammalian Cells
- Rabbit
- NS0
- Wheat Germ
- In Vitro Cell Free System
- Yeast
- His
- Avi
- Non
- Fc
- GST
- DDK
- Myc
- GFP
Background
What is MOG protein?
MOG gene (myelin oligodendrocyte glycoprotein) is a protein coding gene which situated on the short arm of chromosome 6 at locus 6p22. MOG is a component of the central nervous system (CNS) myelin sheaths, primarily found in the brain, spinal cord, and optic nerves. Though its exact function is not fully understood, MOG is thought to serve as a cell surface receptor or a cell adhesion molecule. It belongs to the immunoglobulin superfamily and plays a role in the formation, maintenance, and disintegration of myelin sheaths. The MOG protein is consisted of 247 amino acids and MOG molecular weight is approximately 28.2 kDa.
What is the function of MOG protein?
MOG protein is a component of the central nervous system (CNS) myelin sheaths, primarily found in the brain, spinal cord, and optic nerves. Although its precise function is not completely understood, MOG is believed to act as a cell surface receptor or a cell adhesion molecule, playing a role in the formation, maintenance, and breakdown of myelin sheaths. It is part of the immunoglobulin superfamily and is situated on the outer layer of the myelin sheaths, making it a potential target for autoimmune attacks. MOG's location exposes it to antibodies and T-cell responses, which can lead to demyelination, a process characteristic of inflammatory diseases such as MOG-associated disease (MOGAD). The protein's involvement in immune responses highlights its importance in the pathogenesis of demyelinating disorders and its role in immune system interactions within the CNS.
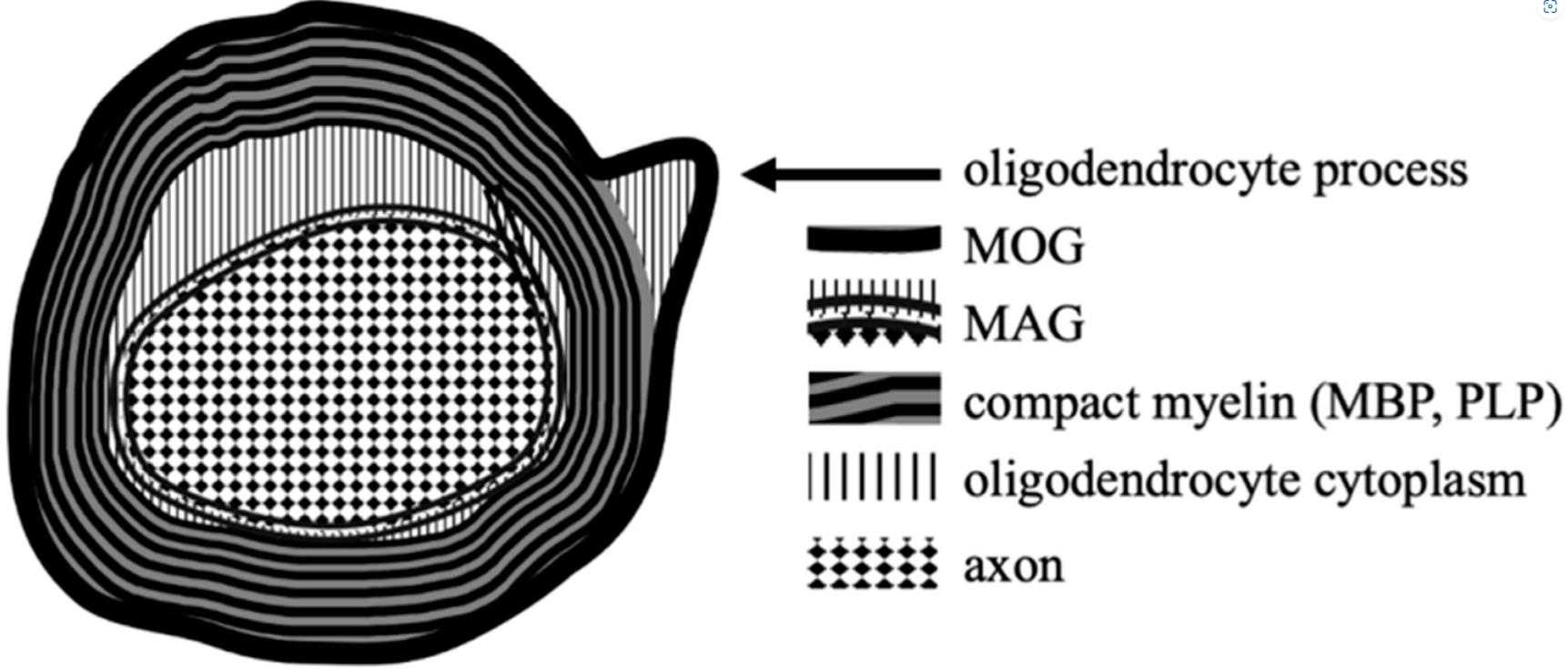
Fig1. The locations of MOG and other myelin proteins on oligodendrocytes within CNS. (Wojciech Ambrosius, 2020)
MOG related signaling pathway
The MOG (Myelin Oligodendrocyte Glycoprotein) protein plays a crucial role in the stability and maintenance of myelin sheaths in the central nervous system. While MOG itself does not directly participate in signaling pathways, its interactions with other molecules can trigger signaling cascades. For instance, binding of MOG by autoantibodies in demyelinating diseases like multiple sclerosis can activate immune response pathways, including the complement system and Fc receptor-mediated phagocytosis. Additionally, disruption of MOG can lead to activation of apoptotic pathways in oligodendrocytes, contributing to myelin damage and neurodegeneration. These interactions underscore the importance of MOG in maintaining myelination and preventing neuroinflammatory responses.
MOG related diseases
MOG is a protein critical for the stability and function of myelin sheaths in the central nervous system. Dysregulation or autoimmune responses targeting MOG can lead to various neurological diseases. For instance, mutations in MOG are associated with congenital white matter disorders, leading to hypomyelination and neurodevelopmental deficits. Moreover, MOG is a major target in demyelinating diseases like multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD), where autoantibodies against MOG trigger inflammatory responses, causing myelin damage and neurological impairments. These conditions highlight the importance of MOG in maintaining myelination and preventing autoimmune-mediated neurodegeneration.
Bioapplications of MOG
In terms of bioapplications, MOG and its associated antibodies are crucial for diagnosing and researching demyelinating diseases. MOG-IgG antibody testing, typically performed using a cell-based assay, is a standard procedure for confirming MOGAD. Additionally, understanding the role of MOG in disease pathogenesis aids in the development of targeted therapies and provides insights into the immune system's interaction with the CNS. Research on MOG and its antibodies also contributes to the differentiation of MOGAD from other similar conditions like multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD), which is critical for appropriate patient management.
Case Study
Case Study 1: Silke Kinzel, 2016
This study reveals that antibodies (Abs) against the myelin oligodendrocyte glycoprotein (MOG) can initiate an encephalitogenic immune response by targeting MOG to myeloid antigen-presenting cells (APCs), even in the absence of B cells. In a transgenic mouse model, MOG-reactive Abs were sufficient to induce spontaneous experimental autoimmune encephalomyelitis (EAE) when mice had endogenous MOG-recognizing T cells. Adoptive transfer studies confirmed that anti-MOG Abs activated and expanded peripheral MOG-specific T cells in an Fc-dependent manner, leading to EAE. In vitro, anti-MOG Abs enabled Fc-mediated APC recognition and presentation of MOG, activating naïve T cells to differentiate into an encephalitogenic phenotype. Translational experiments with Abs from patients with CNS inflammation showed similar results.
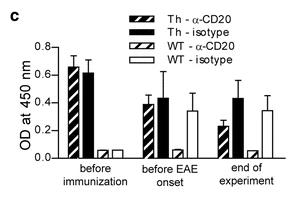
Fig1. Anti-mMOG IgG Ab serum levels determined by ELISA.
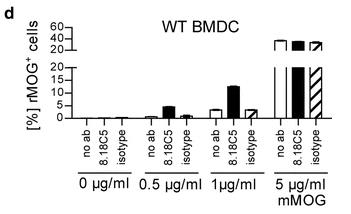
Fig2. Phagocytosis of mMOG by WT BMDC.
Case Study 2: Louise H Boyle, 2007
Though myelin oligodendrocyte glycoprotein (MOG) is considered a potential autoantigen in multiple sclerosis, its specific role is unclear. The MOG gene gives rise to at least nine protein isoforms through alternative mRNA splicing. This study examined six of these isoforms after cloning them into mammalian expression vectors and observed their sub-cellular localization and membrane trafficking using confocal microscopy. Here two full-length isoforms (25.6 and 25.1) were present on the cell surface, while three spliced forms (22.7, 21.0, and 20.5) were more intracellular, localizing to the endoplasmic reticulum and endosomes. The 16.3 isoform, lacking a transmembrane domain, was secreted. Variations in MOG's sub-cellular localization may significantly affect receptor-ligand interactions and the protein's function, with different isoforms potentially exposing immunogenic epitopes within the CNS.
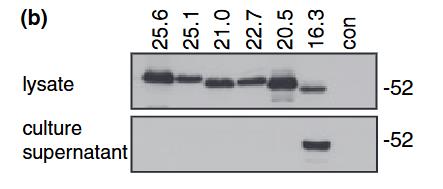
Fig3. Western blots of cellular lysates and culture supernatants of HeLa cells transiently expressing the MOG proteins tagged at the C-terminus with GFP.
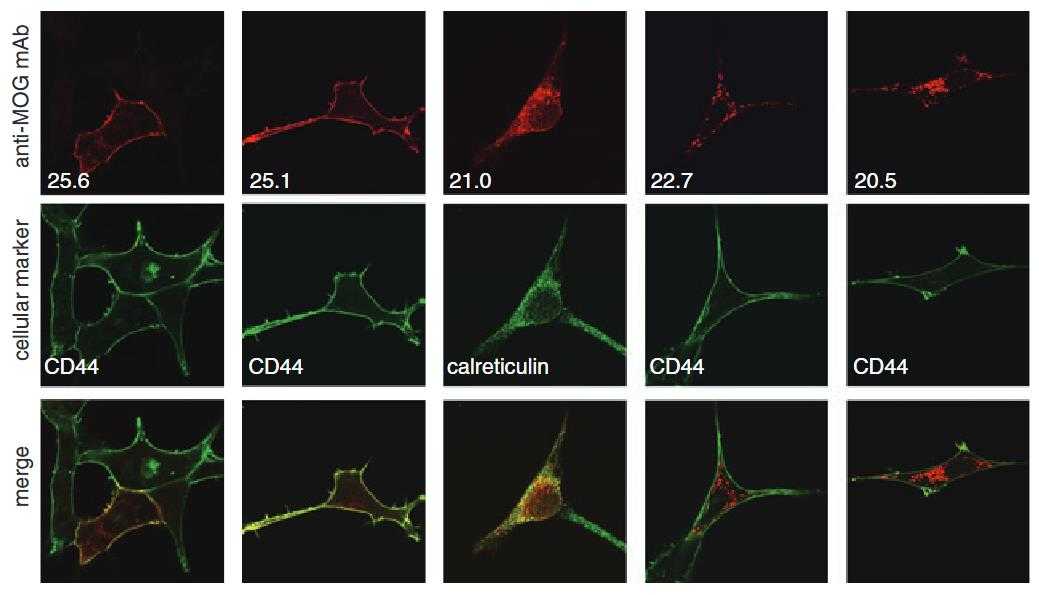
Fig4. Myelin oligodendrocyte glycoprotein (MOG) isoforms exhibit distinct cellular distribution in MO3.13 oligodendrocyte cells.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (MOG-041H)
.
.jpg)
Fig2. SDS-PAGE (MOG-633H)
Involved Pathway
MOG involved in several pathways and played different roles in them. We selected most pathways MOG participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with MOG were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
MOG has several biochemical functions, for example, virus receptor activity. Some of the functions are cooperated with other proteins, some of the functions could acted by MOG itself. We selected most functions MOG had, and list some proteins which have the same functions with MOG. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| virus receptor activity | TNFRSF14,CLEC5A,PVRL1,CD80,TFRC,HYAL2,PVRL2,HLA-DRB1,TNFRSF4,CR1 |
Interacting Protein
MOG has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with MOG here. Most of them are supplied by our site. Hope this information will be useful for your research of MOG.
OTUD4
Resources
Related Services
Related Products
References
- Probstel, AK; Rudolf, G; et al. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. JOURNAL OF NEUROINFLAMMATION 12:-(2015).
- Aktas, O; et al. Collateral benefit: the comeback of MOG antibodies as a biomarker in neurological practice. JOURNAL OF NEUROLOGY NEUROSURGERY AND PSYCHIATRY 86:243-243(2015).



