Recombinant Human Histidine-Rich Glycoprotein
| Cat.No. : | HRG-332H |
| Product Overview : | Recombinant human Heregulin (HRG) protein was expressed inE. coli. MW= 44kDa (full length) and 30kDa (extracellular domain). |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | Non |
| Description : | Heregulin (HRG) is a glycoprotein of 44kDa which binds to c-erbB-3 and c-erbB-4 receptors with low and high affinities, respectively. Heregulins constitute a family of mosaic proteins comprising of at least 12 members. They contain certain recognizable domains which include: an amino terminal (N-ter) region; an immunoglobulinlike motif (Ig); a glycosylated spacer sequence (glyco); an EGF-like motif (EGF), which is subdivided into α and β isoforms; a juxtamembrane region subdivided into isoforms 1-3; a hydrophobic transmembrane domain (TM); a common cytoplasmic tail (Cyto); and a variable-length cytoplasmic tail (a-c). Using this nomenclature, several isoforms of HRG are designated as HRG-α1, HRG-α2, HRG-α3, HRG-β1, HRG-β2, HRG-β3, HRG-α2b, etc. |
| Comments : | In SDS-PAGE, the purified recombinant Heregulin α1 protein appears as a single band at 30kDa. Heregulin α1 protein modulates the proliferation and differentiation of cultured mammary cells and stimulates the tyrosine kinase activity of ErbBs. |
| Applications And Suggested Dilutions : | Western Blotting, Stimulation of Tyrosine Kinase Activity of Erbs (Protein at 5nM); Cell Proliferation and Differentiation (Protein at 5nM: dilute 5μg of stock protein to 30ml of culture medium). The optimal dilution for a specific application should be determined by the investigator. |
| Positive Control : | T47-D breast cancer cells. |
| Supplied As : | Purified Heregulin α1 is packaged in physiological saline, pH 7.4, with 0.2% BSA. Note that this product is supplied without Sodium azide or any other preservative. |
| Characterization : | On SDS-PAGE commassie blue stained gel, the purified recombinant protein shows a band at 45 kDa. |
| Storage And Stability : | Store vial at -20°C to -70°C. When stored at the recommended temperature, this protein is stable for 24 months. |
| Full Length : | Full L. |
| Gene Name | HRG histidine-rich glycoprotein [ Homo sapiens ] |
| Synonyms | HRG; histidine-rich glycoprotein; HPRG; HRGP; DKFZp779H1622; thrombophilia due to elevated HRG; histidine-proline rich glycoprotein; Histidine-proline-rich glycoprotein |
| Gene ID | 3273 |
| mRNA Refseq | NM_000412 |
| Protein Refseq | NP_000403 |
| UniProt ID | P04196 |
| Chromosome Location | 3q27 |
| MIM | 142640 |
| Pathway | Hemostasis |
| Function | cysteine-type endopeptidase inhibitor activity; heparin binding; serine-type endopeptidase inhibitor activity |
| ◆ Recombinant Proteins | ||
| HRG-7862H | Recombinant Human HRG protein, His & GST-tagged | +Inquiry |
| Hrg-967M | Recombinant Mouse Hrg Protein, His-tagged | +Inquiry |
| HRG-425R | Recombinant Rat HRG Protein, His-tagged | +Inquiry |
| HRG-4707H | Recombinant Human HRG Protein, Myc/DDK-tagged, C13 and N15-labeled | +Inquiry |
| HRG-799HFL | Recombinant Full Length Human HRG Protein, C-Flag-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| HRG-2790HCL | Recombinant Human HRG cell lysate | +Inquiry |
Structure and Interactions of a Dimeric Variant of sHIP, a Novel Virulence Determinant of Streptococcus pyogenes
Journal: Frontiers in Microbiology PubMed ID: 26903974 Data: 2016/2/5
Authors: Carl Diehl, Magdalena Wisniewska, Mats Wikstr?m
Article Snippet:Subsequently, the bacteria were diluted to a concentration of 2 × 10 6 cfu/ml in 10 mM MES buffer, pH 5.5, containing 5 mM glucose.Subsequently, the bacteria were diluted to a concentration of 2 × 10 6 cfu/ml in 10 mM MES buffer, pH 5.5, containing 5 mM glucose.. Fifty microliters of the bacterial solution was incubated with recombinant His-tagged HRG (Creative BioMart) at a concentration of 0.45 μM together with various concentrations of protein sHIPwt or protein sHIPqp for 40 min at 37°C.. Serial dilutions of the incubation mixtures were plated on TH agar, plates were incubated over night at 37°C and the number of cfu's were determined.Serial dilutions of the incubation mixtures were plated on TH agar, plates were incubated over night at 37°C and the number of cfu's were determined.
Functional and Structural Properties of a Novel Protein and Virulence Factor (Protein sHIP) in Streptococcus pyogenes
Journal: The Journal of Biological Chemistry PubMed ID: 24825900 Data: 2014/6/27
Authors: Magdalena Wisniewska, Lotta Happonen, Mats Wikstr?m
Article Snippet:Analysis of the molecular interactions between sHIP and HRG, streptococcal protein G, and human ubiquitin, respectively, was performed on a Biacore T200 instrument (GE Healthcare).Analysis of the molecular interactions between sHIP and HRG, streptococcal protein G, and human ubiquitin, respectively, was performed on a Biacore T200 instrument (GE Healthcare).. HRG (Creative BioMart), protein G (Sigma-Aldrich), or ubiquitin (Boston Biochem) was immobilized on a CM3 sensor chip using standard amine coupling at pH 5.0 in 10 m m acetate buffer at two different immobilization levels (1000 and 2000 response units).. All binding experiments were performed at 25 °C in Dulbecco's PBS buffer, pH 7.3 (Biowest) at two flow rates, 30 and 60 μl/min.All binding experiments were performed at 25 °C in Dulbecco's PBS buffer, pH 7.3 (Biowest) at two flow rates, 30 and 60 μl/min.
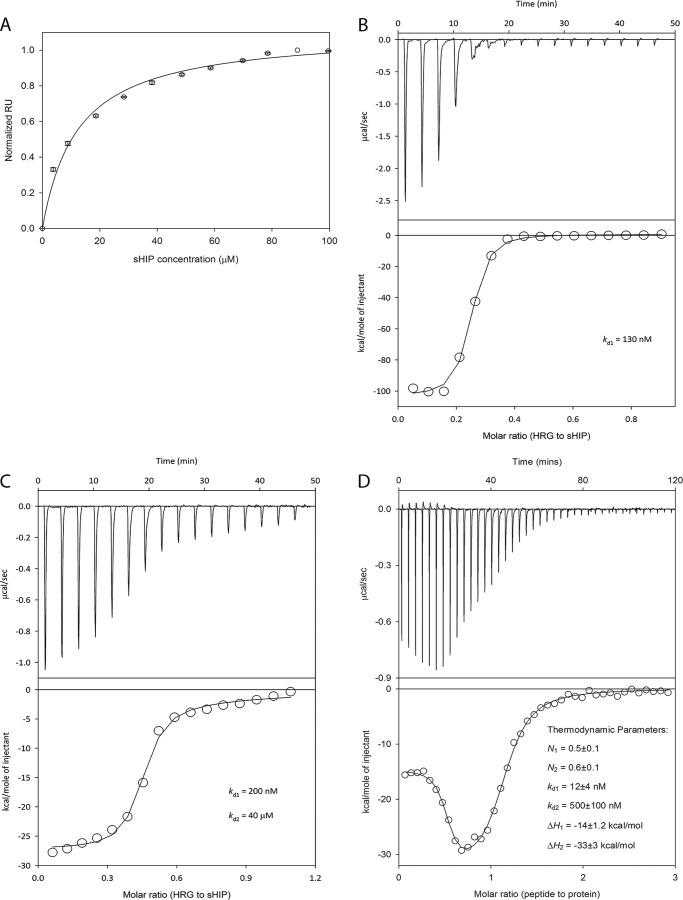
Biophysical studies of the interaction between sHIP and intact
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All HRG Products
Required fields are marked with *
My Review for All HRG Products
Required fields are marked with *



