Recombinant Human CAT protein(Ala2-Leu527), His-tagged
| Cat.No. : | CAT-2500H |
| Product Overview : | Recombinant Human CAT (P04040) (Ala 2-Leu 527) was expressed in Insect Cells, with a polyhistidine tag at the N-terminus. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | Insect Cells |
| Tag : | His |
| Protein Length : | 2-527 a.a. |
| Form : | Lyophilized from sterile 50mM Tris, 100mM NaCl, pH 8.0, 10% gly. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human CAT consists of 545 amino acids and predicts a molecular mass of 61.9 kDa. It migrates as an approximately 60 kDa band in SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 EU per μg of the protein as determined by the LAL method |
| Purity : | > 80 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | CAT catalase [ Homo sapiens ] |
| Official Symbol | CAT |
| Synonyms | CAT; catalase; MGC138422; MGC138424; |
| Gene ID | 847 |
| mRNA Refseq | NM_001752 |
| Protein Refseq | NP_001743 |
| MIM | 115500 |
| UniProt ID | P04040 |
| ◆ Recombinant Proteins | ||
| CAT-0865H | Recombinant Human CAT Protein (Met1-Leu527), His tagged | +Inquiry |
| CAT-1252M | Recombinant Mouse CAT Protein, His (Fc)-Avi-tagged | +Inquiry |
| CAT-1609H | Recombinant Human CAT Protein (Full Length), N-His tagged | +Inquiry |
| CAT-2500H | Recombinant Human CAT protein(Ala2-Leu527), His-tagged | +Inquiry |
| CAT-1600H | Recombinant Human CAT Protein (Ala2-Leu527) | +Inquiry |
| ◆ Native Proteins | ||
| CAT-21H | Native Human Catalase Protein | +Inquiry |
| CAT-1646H | Native Human Catalase Protein | +Inquiry |
| CAT-1187B | Native Bovine Catalase | +Inquiry |
| CAT-101B | Active Native Bovine CAT | +Inquiry |
| CAT-5276H | Native Human, Catalase | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| CAT-515HCL | Recombinant Human CAT cell lysate | +Inquiry |
Case 1: González-Sánchez MI, et al. Arch Biochem Biophys. 2011
Hydrogen peroxide triggers a redox cycle between methemoglobin and ferrylhemoglobin, causing protein inactivation and oxygen production. This study analyzed how methemoglobin behaves like catalase with H2O2, revealing unusual kinetics tied to protein concentration. Superoxide scavengers increased activity slightly, suggesting mostly biocatalytic oxygen production. The proposed mechanism involves competing reactions affecting hemoglobin radicals, and simulations confirmed that methemoglobin’s catalase-like activity primarily protects it from H2O2-induced inactivation.
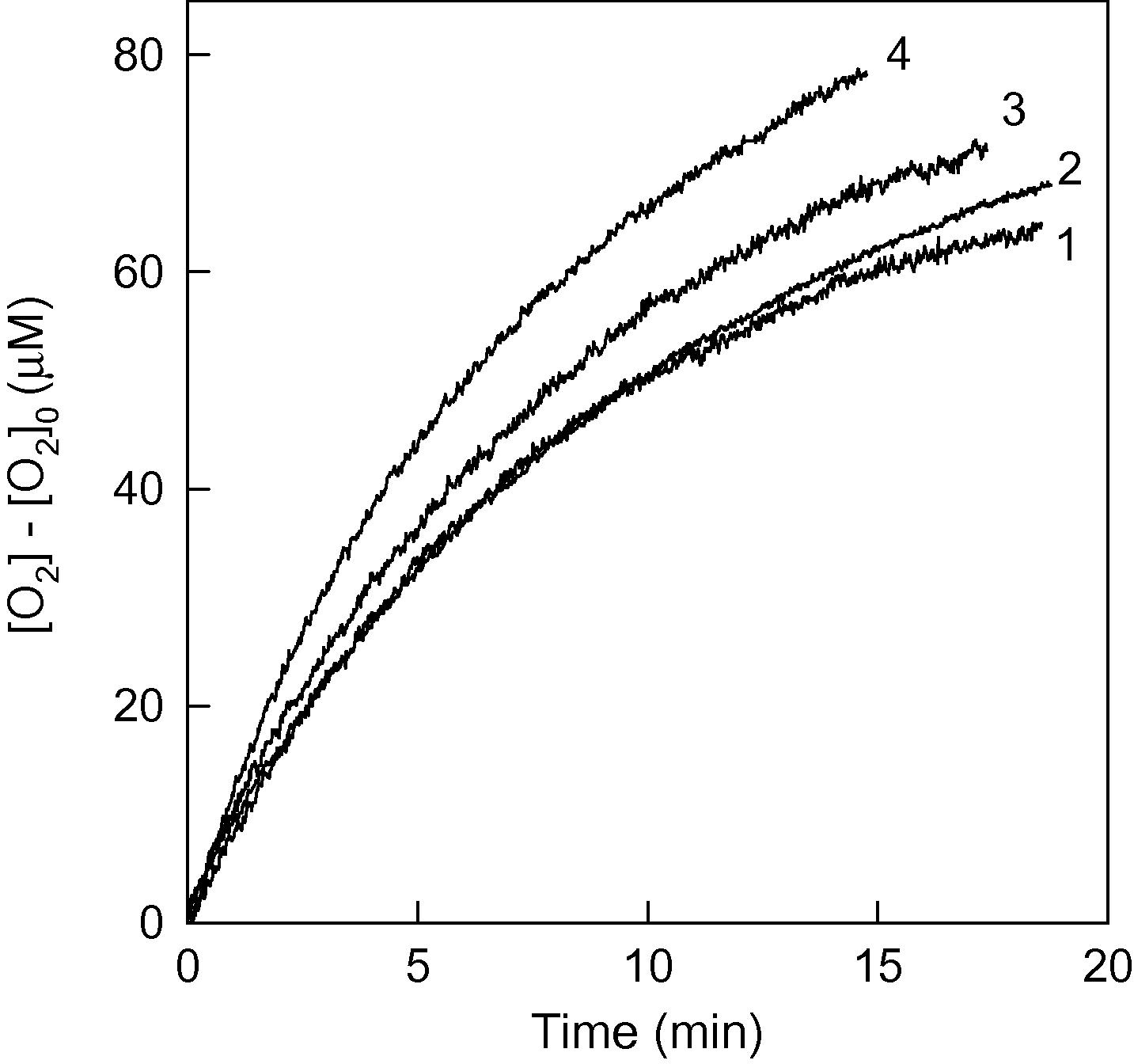
Fig1. The effects of the different superoxide scavengers on the catalase-like oxygen production catalyzed by metHb.
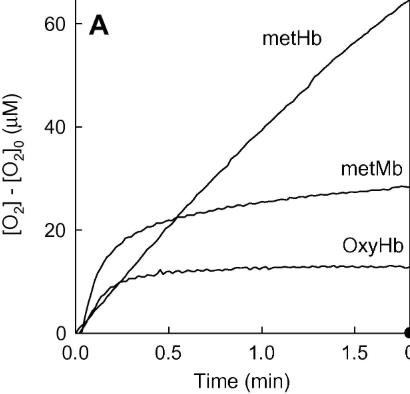
Fig2. Time course of O2 production in a catalase-like reaction of metHb (7.5 μM), oxyHb (5.3 μM) and metMb (9.9 μM) in the presence of [H2O2]0 = 2 mM.
Case 2: Lee JN, et al. Biochem Biophys Res Commun. 2018
Peroxisomes play a vital role in cellular metabolism and are controlled by pexophagy, a specific type of autophagy. Catalase, an important enzyme in peroxisomes, breaks down reactive oxygen species (ROS). However, its involvement in pexophagy had not been explored before. This study found that inhibiting catalase, either genetically or with 3-aminotriazole during nutrient deprivation, reduced peroxisomes and key protein levels, increased ROS, and induced NBR1-dependent autophagy. The antioxidant N-acetyl-l-cysteine stopped ROS accumulation and pexophagy, revealing catalase's significant role in managing peroxisomes under nutrient stress.
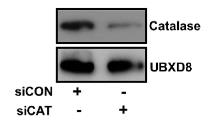
Fig1. Whole cell lysate from HepG2 cells was analyzed for catalase expression by immunoblotting.
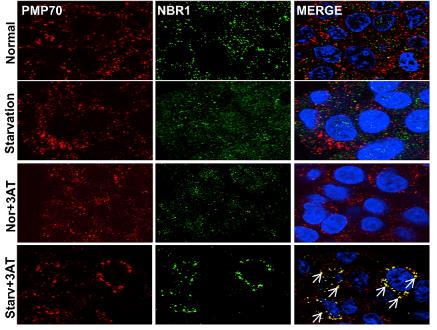
Fig2. HepG2 cells were treated as indicated, immunostained with PMP70 (red), NBR1 (green) and DAPI (blue), and observed under a confocal microscopy.
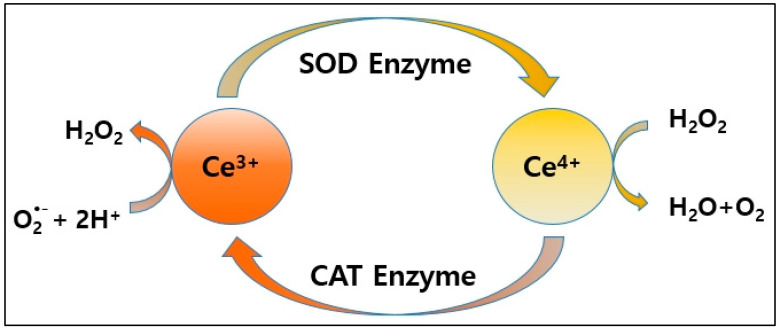
Fig1. Nanoceria exerts antioxidant effects by scavenging free radicals by mimicking the activities of SOD and CAT. (Da-Long Dong, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All CAT Products
Required fields are marked with *
My Review for All CAT Products
Required fields are marked with *
Inquiry Basket


