Recombinant Human AMTN protein(Met1-Gln209), mFc-tagged
| Cat.No. : | AMTN-221H |
| Product Overview : | Recombinant Human AMTN (NP_997722.1) (Met1-Gln209) was expressed in HEK293 with the Fc region of Mouse IgG1 at the C-terminus. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | HEK293 |
| Tag : | mFc |
| Protein Length : | Met1-Gln209 |
| Form : | Lyophilized from sterile 50mM Tris, 100mM NaCl, 10% glycerol,0.5mM EDTA, pH 8.5. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human AMTN/mFc comprises 427 amino acids and has a predicted molecular mass of 46.2 kDa. The apparent molecular mass of the monomer is approximately 54 kDa in SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 EU per μg of the protein as determined by the LAL method. |
| Purity : | > 78 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | AMTN amelotin [ Homo sapiens ] |
| Official Symbol | AMTN |
| Synonyms | UNQ689 |
| Gene ID | 401138 |
| mRNA Refseq | NM_212557.2 |
| Protein Refseq | NP_997722.1 |
| MIM | 610912 |
| UniProt ID | Q6UX39 |
| ◆ Recombinant Proteins | ||
| AMTN-1023H | Recombinant Human AMTN | +Inquiry |
| AMTN-9627H | Recombinant Human AMTN, GST-tagged | +Inquiry |
| AMTN-221H | Recombinant Human AMTN protein(Met1-Gln209), mFc-tagged | +Inquiry |
| AMTN-317R | Recombinant Rat AMTN Protein, His (Fc)-Avi-tagged | +Inquiry |
| AMTN-661R | Recombinant Rat AMTN Protein | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| AMTN-001HCL | Recombinant Human AMTN cell lysate | +Inquiry |
Case 1: Fouillen A, et al. Sci Rep. 2017
A specialized basal lamina (sBL) plays a crucial role in enabling certain epithelial cells to adhere to teeth. Unlike traditional basal lamina, sBL is unique because it lacks type IV and VII collagens and is rich in laminin-332. It also contains three newly identified components: amelotin (AMTN), odontogenic ameloblast-associated (ODAM), and secretory calcium-binding phosphoprotein proline-glutamine-rich 1 (SCPPPQ1). This study aimed to investigate the structural organization of the sBL. Through fluorescence and immunogold labeling techniques, it was found that these proteins co-localize within the sBL. Analysis of the distribution patterns showed ODAM leaning towards the cell side, while AMTN and SCPPPQ1 were closer to the tooth surface. Further experiments, including bacterial two-hybrid analysis, co-immunoprecipitation, and various microscopy methods, revealed that AMTN, ODAM, and SCPPPQ1 interact to form complex structures. This organization helps form an extracellular matrix that securely anchors epithelial cells to mineralized surfaces, which is vital for attaching gingival epithelial cells to teeth, offering a protective barrier against bacterial invasion.
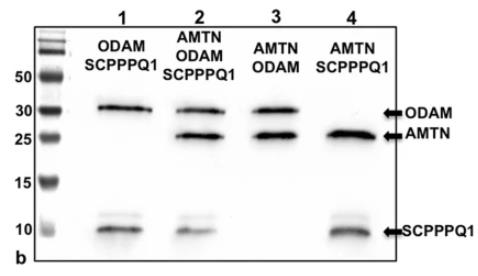
Fig1. These interactions are confirmed by co-immunoprecipitation followed by Western blotting.
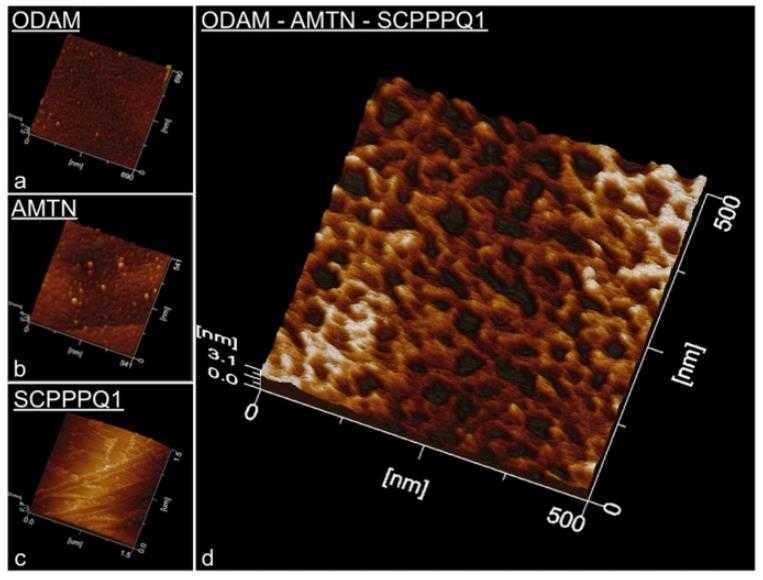
Fig2. Atomic force microscope characterization of AMTN, ODAM and SCPPPQ1 proteins.
Case 2: Ikeda Y, et al. Cell Mol Bioeng. 2022
Periodontitis leads to the degradation of tissues supporting teeth, including the alveolar bone. In dental tissue regenerative therapy, barrier membranes are employed, but they often face challenges like unstable membrane integrity and insufficient promotion of bone mineralization. Amelotin (AMTN), a protein from the enamel matrix, helps in hydroxyapatite formation and growth. This study explored the effects of recombinant human AMTN (rhAMTN) on mineralization and adhesion using a collagen-based system. By integrating rhAMTN into a collagen hydrogel (AMTN gel) and applying it onto dentin surfaces, mineral deposits were significantly enhanced. The AMTN gel induced hydroxyapatite in the collagen matrix, while rhAMTN-coated dentin exhibited mineral deposit formation on its surface. Notably, the AMTN membrane demonstrated localized mineralization, releasing only 1% of rhAMTN. This resulted in a membrane that had more than twice the tensile strength when adhered to dentin compared to a barrier membrane without rhAMTN.
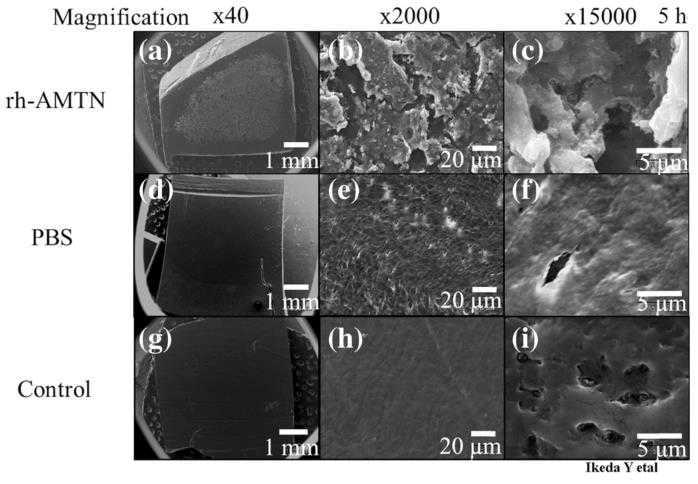
Fig1. SEM images of rhAMTN-treated dentin incubated in SBF buffer.
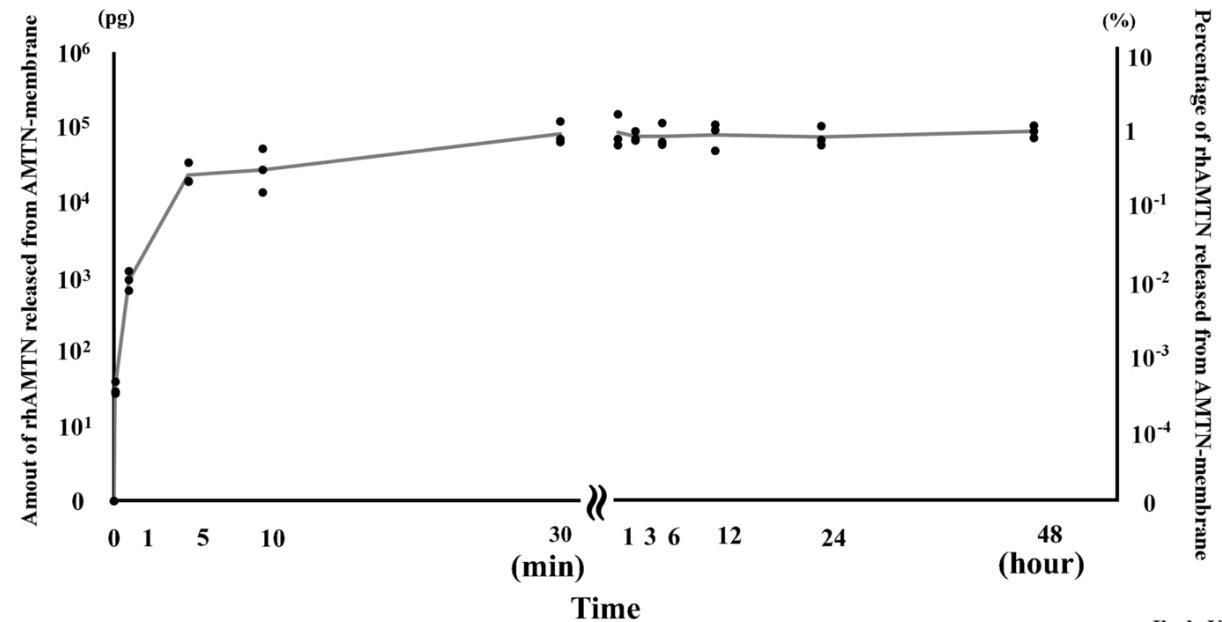
Fig2. Release kinetics of rhAMTN from the AMTN membrane.
Human AMTN protein plays a vital role in the mineralization of enamel, significantly contributing to the maturation and stability of tooth enamel. As technology has progressed, recombinant human AMTN protein has demonstrated extensive potential in both scientific research and industrial applications. In research, recombinant AMTN proteins are employed to better understand the molecular mechanisms of enamel development and to explore new methods for enamel repair and regeneration. These investigations offer a foundation for the innovation of dental materials and treatments.
In the realm of industrial production, the use of recombinant human AMTN protein is growing. It holds significant value in advancing dental materials' research, particularly in improving the biocompatibility and functionality of dental restoration products. Moreover, this protein is pivotal in the development and testing of biologics aimed at enhancing tooth regeneration, marking progress in oral health innovations. These applications not only enhance dental care product quality but also present novel solutions for preventing and treating dental ailments.
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All AMTN Products
Required fields are marked with *
My Review for All AMTN Products
Required fields are marked with *



