Recombinant E. coli udp Protein, 1-253aa, N-6×His tagged
| Cat.No. : | Udp-782E |
| Product Overview : | Recombinant E. coli uDP protein, fused to His-tag at N-terminus, was expressed in E. coli and purified by using conventional chromatography techniques. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | E.coli |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | 1-253aa |
| Description : | Catalyzes the reversible phosphorylytic cleavage of uridine to uracil and ribose-1-phosphate. Shows weak activity towards deoxyuridine and thymidine. The produced molecules are then utilized as carbon and energy sources or in the rescue of pyrimidine bases for nucleotide synthesis (Probable). |
| Form : | Liquid |
| Bio-activity : | > 20,000 pmol/min/μg, and is defined as the amount of enzyme that catalyze the reduction 1.0 pmole of uridine presence of phosphate per minute at pH 7.5 at 25 centigrade. |
| Molecular Mass : | 29.3 kDa (273aa) confirmed by MALDI-TOF |
| AA Sequence : | MGSSHHHHHHSSGLVPRGSHMSKSDVFHLGLTKNDLQGATLAIVPGDPDRVEKIAALMDKPVKLASHREFTTWRAELDGKPVIVCSTGIGGPSTSIAVEELAQLGIRTFLRIGTTGAIQPHINVGDVLVTTASVRLDGASLHFAPLEFPAVADFECTTALVEAAKSIGATTHVGVTASSDTFYPGQERYDTYSGRVVRHFKGSMEEWQAMGVMNYEMESATLLTMCASQGLRAGMVAGVIVNRTQQEIPNAETMKQTESHAVKIVVEAARRLL |
| Purity : | > 95% by SDS-PAGE |
| Applications : | SDS-PAGE, Enzyme Activity |
| Storage : | Can be stored at +2 to +8 centigrade for 1 week. For long term storage, aliquot and store at -20 to -80 centigrade. Avoid repeated freezing and thawing cycles. |
| Concentration : | 1 mg/mL (determined by Bradford assay) |
| Storage Buffer : | 20mM Tris-HCl buffer (pH 8.0) containing 10% glycerol, 1mM DTT, 50mM NaCl |
| References : | 1. Burling F.T., et al. (2003) Acta Crystallogr. 59:73-76 2. Walton L., et al (1989) Nucleic Acids Res. 17(16):6741. |
| Gene Name | udp uridine phosphorylase [ Escherichia coli str. K-12 substr. MG1655 ] |
| Official Symbol | udp |
| Synonyms | udp; uridine phosphorylase; ECK3825; uridine phosphorylase; EC 2.4.2.3 |
| Gene ID | 948987 |
| Protein Refseq | NP_418275 |
| UniProt ID | P12758 |
| ◆ Recombinant Proteins | ||
| UDP-1285S | Recombinant Satsuma mandarin UDP protein, His&Myc-tagged | +Inquiry |
| CEZ91P | Active Recombinant Salmonella typhimurium udp | +Inquiry |
| Udp-782E | Recombinant E. coli udp Protein, 1-253aa, N-6×His tagged | +Inquiry |
Case 1: Feng Y, et al. Biotechnol Adv. 2020
Plant glycosides have caught the eye of many industries because they can be super useful. When these compounds go through glycosylation, they become more soluble, biologically active, and stable. This means they can be handy as food additives, in cosmetics, health products, and even as antiseptics and anti-cancer drugs. As the demand keeps growing, new bio-fermentation techniques are popping up. This review zeroes in on how recombinant microbes are used to synthesize plant glycosides, with UDP-sugars as the starting point. It talks about different ways to amp up glycoside production, focusing on four main strategies like overexpressing genes, recycling UDP-sugars, mixed fermentation, and using carbon efficiently. Plus, it highlights the potential and perks of these methods, offering tips on crafting high-yield strains for microbial production. It also touches on the challenges faced in glycoside biosynthesis and shares insights on boosting yields in the future.
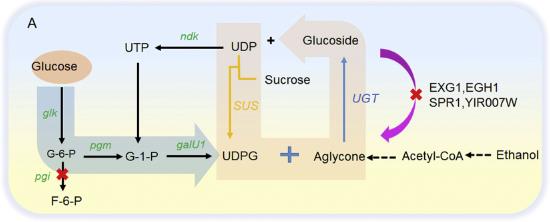
Fig1. Regulation of UDPG synthetic pathway to enhance precursor supply.
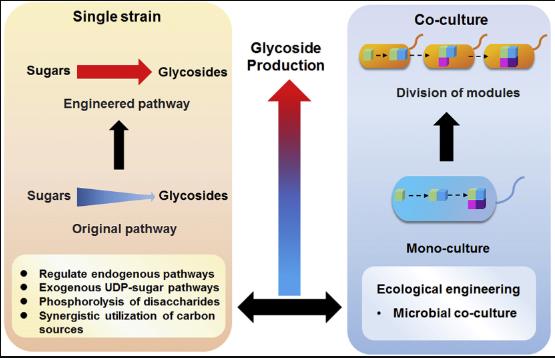
Fig2. Schematic representation of engineering strategies of chassis and microbial ecology in improving UDP-sugar supply for glycoside biosynthesis.
Case 2: Zhuang JD, et al. JACS Au. 2024
Researchers are diving into making UDP-d-xylose, a key player in crafting drugs like heparin. By tapping into a newly found UDP-xylose route, they created some cool enzyme combos from Solitalea canadensis that turn d-xylose straight into UDP-d-xylose. In this study, researchers played around with different protein mixes to see how they work in E. coli and came up with a powerful two-in-one enzyme. This top-performing version included an NH2-GSGGGSGHM-COOH linker and managed best at a pH of 7.0 and a temperature of 30°C. With ideal conditions set, this enzyme showed impressive results, even doing better with ATP and ADP control. Mixing unfused enzymes at a perfect 1:1 ratio also proved key in the process. In short, they crafted an efficient path to produce xylosides, which could be a game-changer for biotech and drugs.
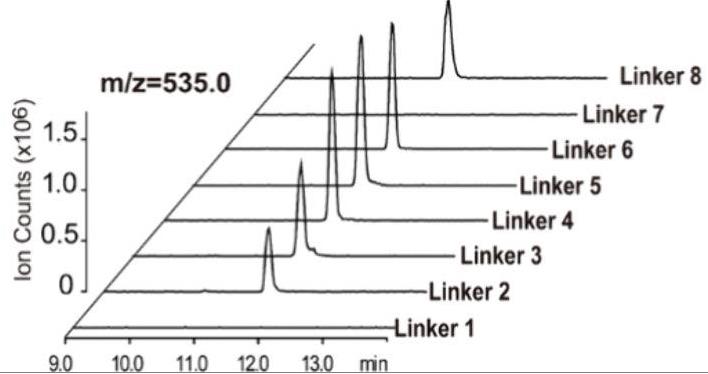
Fig1. LC-ESI-MS profile confirming the presence of UDP-xylose in reaction mixtures containing ScGalK/ScGPUT fusion enzymes.
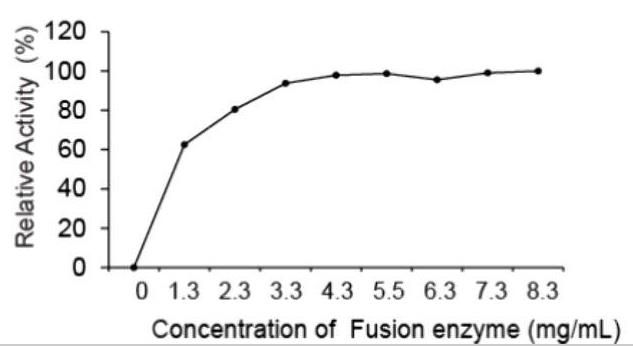
Fig2. TLC results were used for identifying the optimal enzyme concentration for UDP-d-xylose enzymatic synthesis.
Recombinant E. coli UDP protein, particularly the enzyme UDP-glucose dehydrogenase (UGD), is a major player when it comes to making UDP-glucuronate. This compound is vital for building important polysaccharides and glycosaminoglycans in bacteria like E. coli. Basically, UGD helps transform UDP-glucose into UDP-glucuronate, a step that's key for different biological activities, such as producing colanic acid, a kind of exopolysaccharide.
In scientific studies, the recombinant E. coli UDP protein is a go-to tool for digging into how bacteria live and grow. It's really handy for figuring out how bacteria manage certain sugars, aiding researchers in mapping out their metabolic routes. Beyond just understanding, this protein is crucial for crafting new antibacterial medications by zeroing in on what makes bacteria tick. On the industrial front, using E. coli UDP protein can supercharge fermentation, making it a boost for producing biofuels and other bio-based products. So, beyond pushing the boundaries of knowledge, this protein has real-world uses that make a big difference in industry.
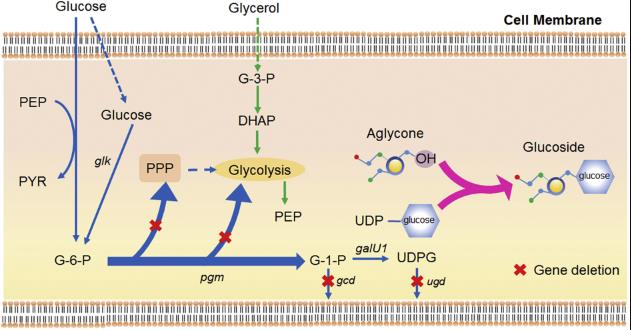
Fig1. Synergistic utilization of glucose and glycerol for the synthesis of glucosides. (Yueyang Feng, 2020)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All Udp Products
Required fields are marked with *
My Review for All Udp Products
Required fields are marked with *
Inquiry Basket


