Recombinant Chicken MYBPC3
| Cat.No. : | MYBPC3-6673C |
| Product Overview : | Recombinant Chicken MYBPC3 full length or partial length protein was expressed. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Chicken |
| Source : | Mammalian Cells |
| Tag : | His |
| Form : | Liquid or lyophilized powder |
| Endotoxin : | < 1.0 eu per μg of the protein as determined by the LAL method. |
| Purity : | >80% |
| Notes : | This item requires custom production and lead time is between 5-9 weeks. We can custom produce according to your specifications. |
| Storage : | Store it at +4 oC for short term. For long term storage, store it at -20 oC~-80 oC. |
| Storage Buffer : | PBS buffer |
| Gene Name | MYBPC3 myosin binding protein C, cardiac [ Gallus gallus (chicken) ] |
| Official Symbol | MYBPC3 |
| Gene ID | 396013 |
| Protein Refseq | NP_990447 |
| UniProt ID | Q90688 |
| ◆ Recombinant Proteins | ||
| MYBPC3-6673C | Recombinant Chicken MYBPC3 | +Inquiry |
| MYBPC3-19H | Recombinant Human MYBPC3 protein, MYC/DDK-tagged | +Inquiry |
| MYBPC3-943H | Recombinant Human MYBPC3 Protein, Myc/DDK-tagged, C13 and N15-labeled | +Inquiry |
| MYBPC3-3565H | Recombinant Human MYBPC3 protein, GST-tagged | +Inquiry |
| MYBPC3-1125H | Recombinant Human MYBPC3 protein, His-tagged | +Inquiry |
Case 1: Mun JY, et al. Proc Natl Acad Sci U S A. 2014
Myosin-binding protein C, known as MyBP-C, is an additional component of the thick filaments in striated muscle and plays a role in regulating the contraction of cardiac muscle. Laboratory experiments indicate that MyBP-C not only attaches to the thick filament at its C-terminus but also has the capacity to connect with actin through its N-terminal regions, thereby influencing the movement of thin filaments. Visual analysis of F-actin decorated with the N-terminal sections of MyBP-C implies that MyBP-C may bind to actin near the low calcium (Ca2+) binding site of tropomyosin, hinting at a potential regulatory role on thin filament activity by disrupting the movement of tropomyosin on actin.
To verify if MyBP-C's binding has an impact on the positioning of tropomyosin, electron microscopy and in vitro motility tests were utilized to investigate the structural and functional implications of N-terminal fragments' interaction with thin filaments. Three-dimensional reconstructions indicate that under low calcium conditions, MyBP-C pushes tropomyosin towards its position associated with high calcium levels, which aligns with the activation of thin filaments observed in the motility assay. Conversely, in a high calcium environment, MyBP-C's influence on the position of tropomyosin is minimal, but it does result in a reduction of the speed at which thin filaments move. A truncated N-terminal fragment did not shift the position of tropomyosin or trigger thin filament activation under low calcium conditions, yet it slowed the sliding of thin filaments to the same extent as the larger fragments.
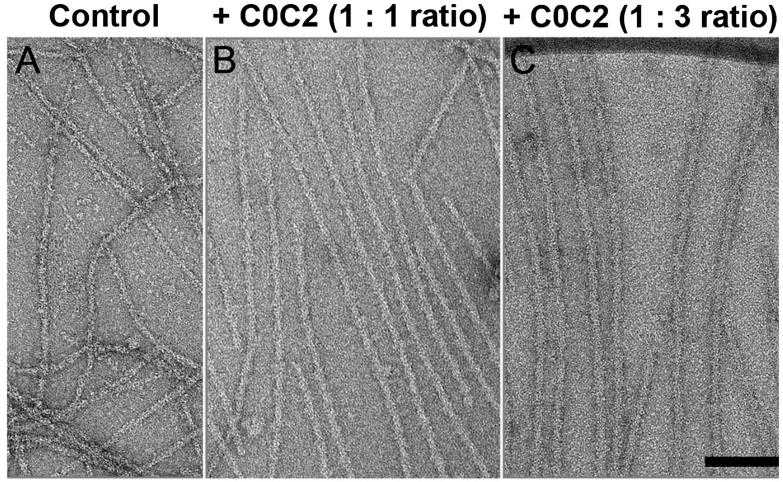
Fig1. Decoration of native thin filaments with C0C2 under low Ca2+ conditions.

Fig2. Effect of N-terminal cMyBP-C fragments on native thin filament sliding in in vitro motility assays.
Case 2: Inchingolo AV, et al. Proc Natl Acad Sci U S A. 2019
The initiation of cardiac muscle contraction is instigated by the binding of calcium to troponin, which sets off a chain of events. The shift in position of tropomyosin, triggered by this binding, allows myosin to attach to actin, leading to the generation of force. In this process of activation, cardiac myosin-binding protein C (cMyBP-C) acts as a regulatory factor. One possible way the N-terminal domains of cMyBP-C modulate this process is by attaching to the actin-thin filament under low calcium conditions, potentially amplifying tropomyosin's movement. To elucidate the precise molecular mechanisms through which cMyBP-C boosts the recruitment of myosin to the actin-thin filament, we employed a fluorescence-based single-molecule microscopy assay. In this assay, researchers visualized the binding of cMyBP-C's N-terminal fragments, labeled with fluorescence, and myosin molecules, labeled with GFP, to actin-thin filaments that were suspended in solution. The attachment of the C0C3 N-terminal fragment of cMyBP-C to the thin filament was observed to increase myosin's connection at low calcium levels. In contrast, under high calcium levels, C0C3 aggregated in clusters, impeding myosin's ability to bind. The dynamic tracking of Cy3-C0C3 molecules bound to the thin filament revealed that these fragments move along the filament before settling into a fixed position.
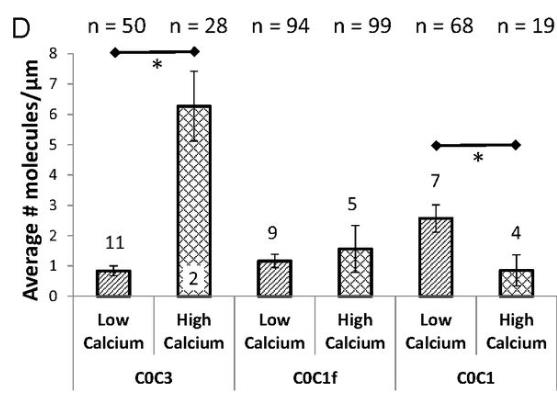
Fig1. Bar chart indicating the average number ± SEM of Cy3-tagged N-terminal cMyBP-C fragments bound per micrometer micrometer of tightrope.
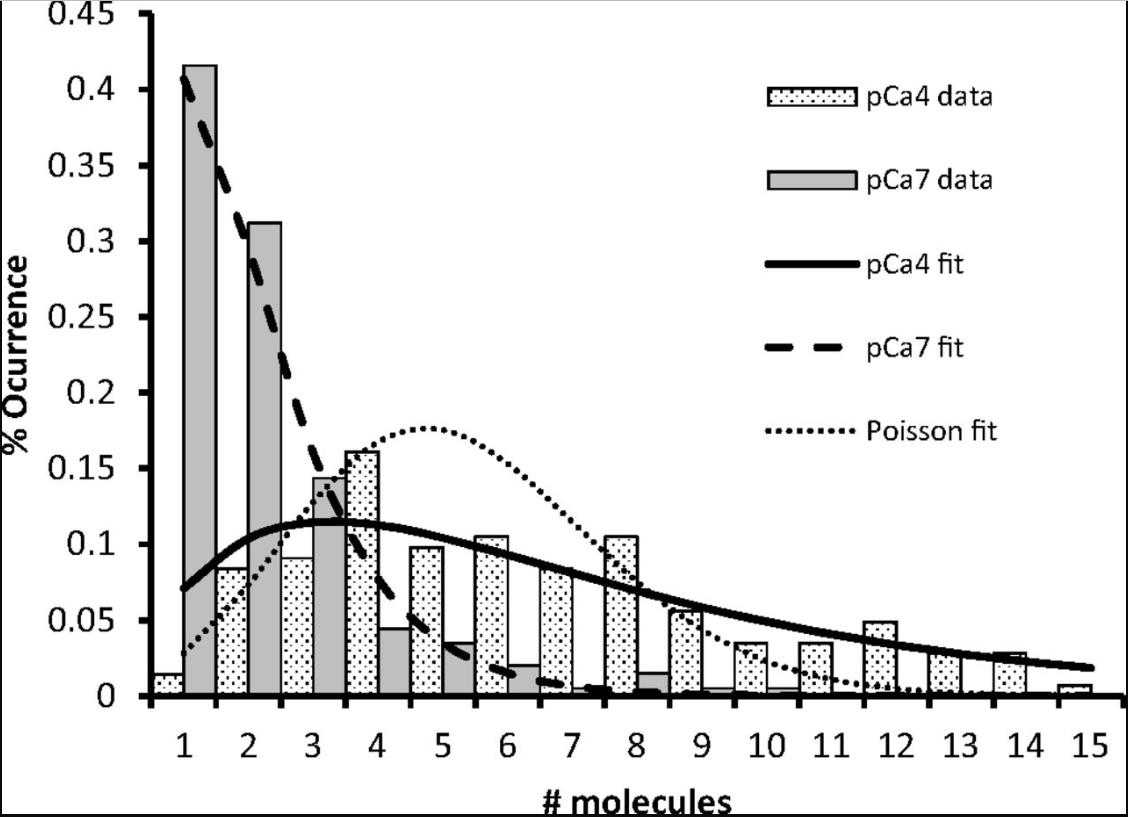
Fig2. Histogram of cMyBP-C cluster sizes.
Chicken MYBPC3 is a protein encoded by the MYBPC3 gene extracted from chicken heart muscle cells. MYBPC3 is a myosin-binding protein that plays an important role in muscle contraction and heart function. By studying the structure and function of Recombinant Chicken MYBPC3, we can gain insight into the mechanisms of muscle contraction and heart function.
Chicken MYBPC3 can be used as a target for drug development, and by studying how it binds to myosin and its mechanism of action, new drugs can be developed to treat muscle diseases and heart diseases. Recombinant Chicken MYBPC3 can be produced in large quantities through genetic engineering techniques for the preparation of recombinant protein drugs or other biological products. Recombinant Chicken MYBPC3 can be used in the food industry, for example as a meat quality modifier to improve the tenderness and taste of meat.
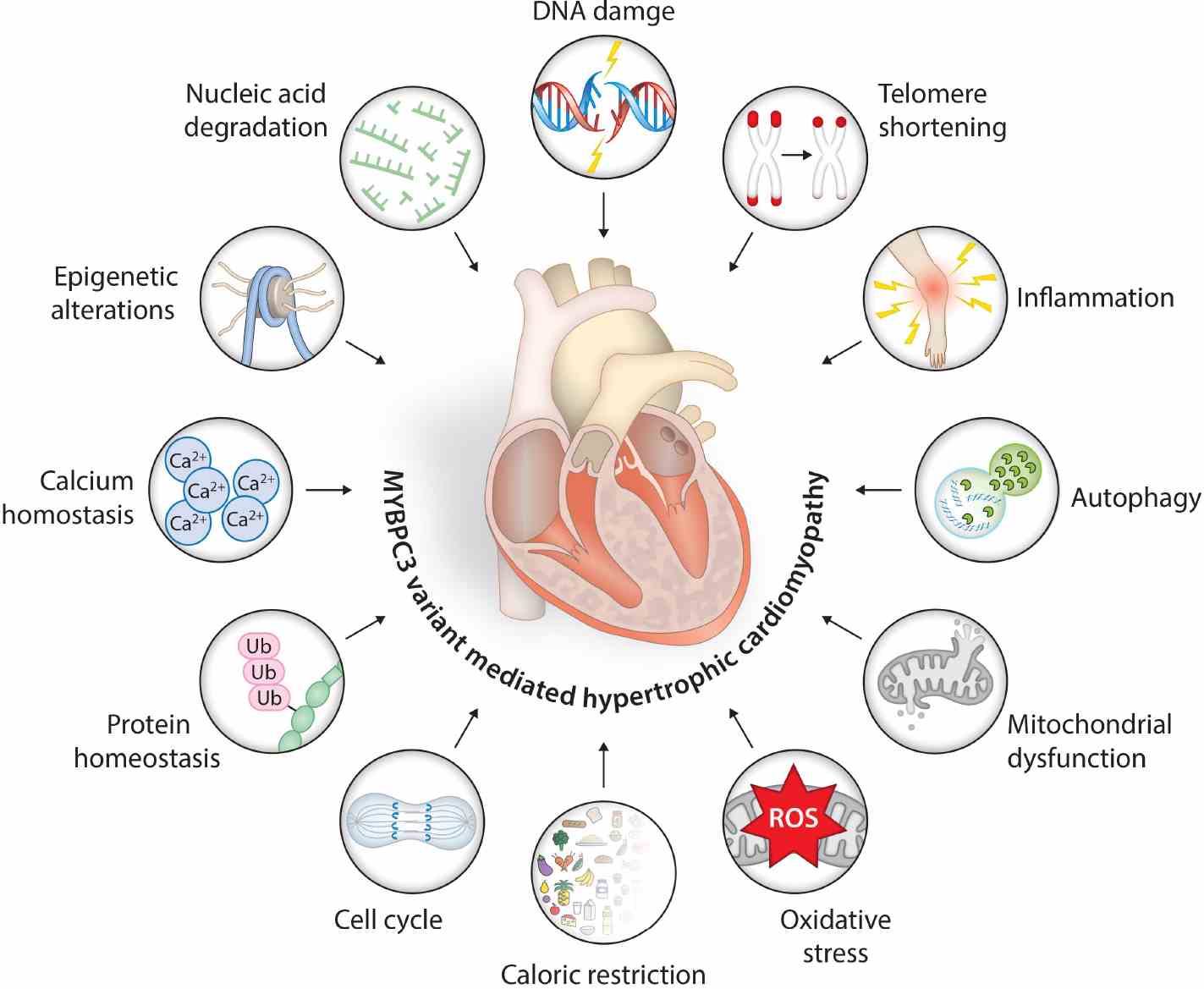
Fig1. Schematic representation of MYBPC3 gene variant-associated molecular events and age-associated hallmarks triggering hypertrophic signals in cardiac muscles. (Kalyani Ananthamohan, 2024)
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All MYBPC3 Products
Required fields are marked with *
My Review for All MYBPC3 Products
Required fields are marked with *


