Native Human HBA2
| Cat.No. : | HBA2-27786TH |
| Product Overview : | Full length native Human Haemoglobin A1c purified from Human erythrocytes; 141 amino acids, MWt 15 kDa. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | Human Erythrocyte |
| Tag : | Non |
| Protein Length : | 141 amino acids |
| Description : | The human alpha globin gene cluster located on chromosome 16 spans about 30 kb and includes seven loci: 5- zeta - pseudozeta - mu - pseudoalpha-1 - alpha-2 - alpha-1 - theta - 3. The alpha-2 (HBA2) and alpha-1 (HBA1) coding sequences are identical. These genes differ slightly over the 5 untranslated regions and the introns, but they differ significantly over the 3 untranslated regions. Two alpha chains plus two beta chains constitute HbA, which in normal adult life comprises about 97% of the total hemoglobin; alpha chains combine with delta chains to constitute HbA-2, which with HbF (fetal hemoglobin) makes up the remaining 3% of adult hemoglobin. Alpha thalassemias result from deletions of each of the alpha genes as well as deletions of both HBA2 and HBA1; some nondeletion alpha thalassemias have also been reported. |
| Form : | Liquid |
| Molecular Mass : | 15.000kDa |
| Storage : | Shipped on dry ice. Upon delivery aliquot and store at -20°C . Avoid freeze / thaw cycles. |
| Storage Buffer : | Proprietary buffer formulation, pH 8.0. |
| Gene Name | HBA2 hemoglobin, alpha 2 [ Homo sapiens ] |
| Official Symbol | HBA2 |
| Synonyms | HBA2; hemoglobin, alpha 2; hemoglobin subunit alpha; alpha globin; alpha-globin; alpha-2 globin; hemoglobin alpha chain; HBH; |
| Gene ID | 3040 |
| mRNA Refseq | NM_000517 |
| Protein Refseq | NP_000508 |
| MIM | 141850 |
| UniProt ID | P69905 |
| Chromosome Location | 16p13.3 |
| Pathway | African trypanosomiasis, organism-specific biosystem; African trypanosomiasis, conserved biosystem; Malaria, organism-specific biosystem; Malaria, conserved biosystem; Selenium Pathway, organism-specific biosystem; |
| Function | contributes_to haptoglobin binding; heme binding; metal ion binding; oxygen binding; oxygen transporter activity; contributes_to peroxidase activity; protein binding; |
| ◆ Recombinant Proteins | ||
| HBA2-29306TH | Recombinant Human HBA2 | +Inquiry |
| HBA2-2040R | Recombinant Rhesus monkey HBA2 Protein, His-tagged | +Inquiry |
| HBA2-856HFL | Recombinant Full Length Human HBA2 Protein, C-Flag-tagged | +Inquiry |
| HBA2-3490HF | Recombinant Full Length Human HBA2 Protein, GST-tagged | +Inquiry |
| HBA2-098H | Recombinant Human HBA2 Protein, MYC/DDK-tagged, C13 and N15-labeled | +Inquiry |
| ◆ Native Proteins | ||
| HBA2-27787TH | Native Human HBA2 | +Inquiry |
| HBA2-27784TH | Native Human HBA2 | +Inquiry |
| HBA2-27786TH | Native Human HBA2 | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| HBA2-5623HCL | Recombinant Human HBA2 293 Cell Lysate | +Inquiry |
Case 1: Ma Q, et al. Front Biosci (Landmark Ed). 2019
Red blood cells (RBCs) encounter exogenous reactive oxygen species in the circulatory system. Thus, researchers evaluated the interactions between different hemoglobin (Hb) subunits and Peroxiredoxin 2 (Prx2). Increment of Diversity with Quadratic Discriminant Analysis (IDQD) is proposed by Quadratic Discriminant Analysis (QDA). Researchers predict that Prx2 may interact with the α, β, and γ subunits of hemoglobin, but not with the δ subunits. To verify these predictions, co-IP was performed. electrospray ionization quadrupole time of flight (ESI-Q-TOF) mass spectrometry, ESI-Q-TOF mass spectrometry, WB and X-ray Absorption Fine Structure (XAFS). The results showed that Prx2 is a part of the β-globulin immunoprecipitation complex present in hemoglobin A, hemoglobin A hemolysis products, hemoglobin A-hemoglobin A2, hemoglobin A-hemolysis product-Hemoglobin A2, and hemoglobin A2, but not in hemoglobin A2 hemolysis products. The addition of Prx2 to hemoglobin A changes the second-layer coordination environment of iron embedded in hemoglobin A.
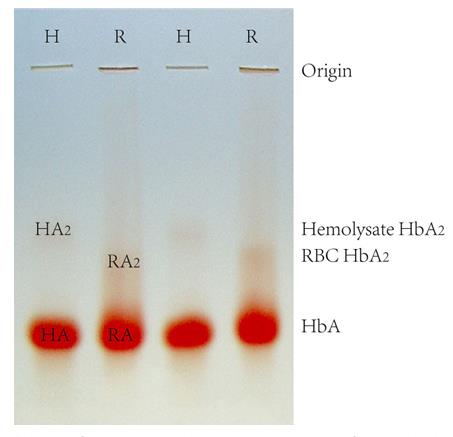
Fig1. Starch-agarose mixed gel electrophoresis of human Hb.
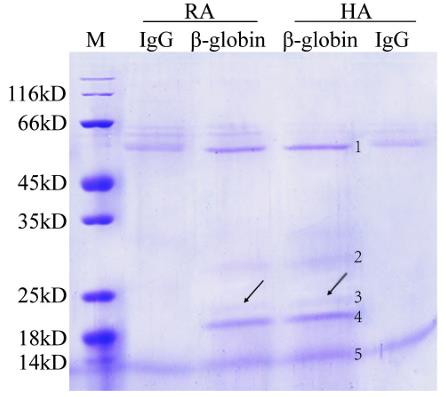
Fig2. Separating Beta-globin immunoprecipitating complexes by SDS-PAGE.
Case 2: Richter F, et al. J Comp Neurol. 2009
This study reports that the expression of hemoglobin chains in mammalian brain neurons is regulated by mitochondrial toxins. Quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis confirmed the expression of Hba-a2 and Hbb in substantia nigra dopamine neurons, striatum gamma-aminobutyric acid (GABA) ergic neurons and cortical pyramidal neurons. Combined in situ hybridization histochemistry and immunohistochemistry using neurospecific nuclear antigen (NeuN) further confirmed the presence of hemoglobin mRNA in neurons in rat brains. Immunohistochemistry identified hemoglobin alpha and beta chains in rats and human brains, and hemoglobin proteins were detected by Western imprinting in rat whole brain tissue as well as in cultures of midbrain neurons, further ruling out the possibility of blood contamination. Systemic administration of the mitochondrial inhibitor rotenone (2 mg/kg/ day, 7 days, subcutaneous injection) significantly reduced Hba-a2 and Hbb mRNA in substantia nigra dopamine neurons, while neuroglobulin or cellular globulin mRNA was unaffected.
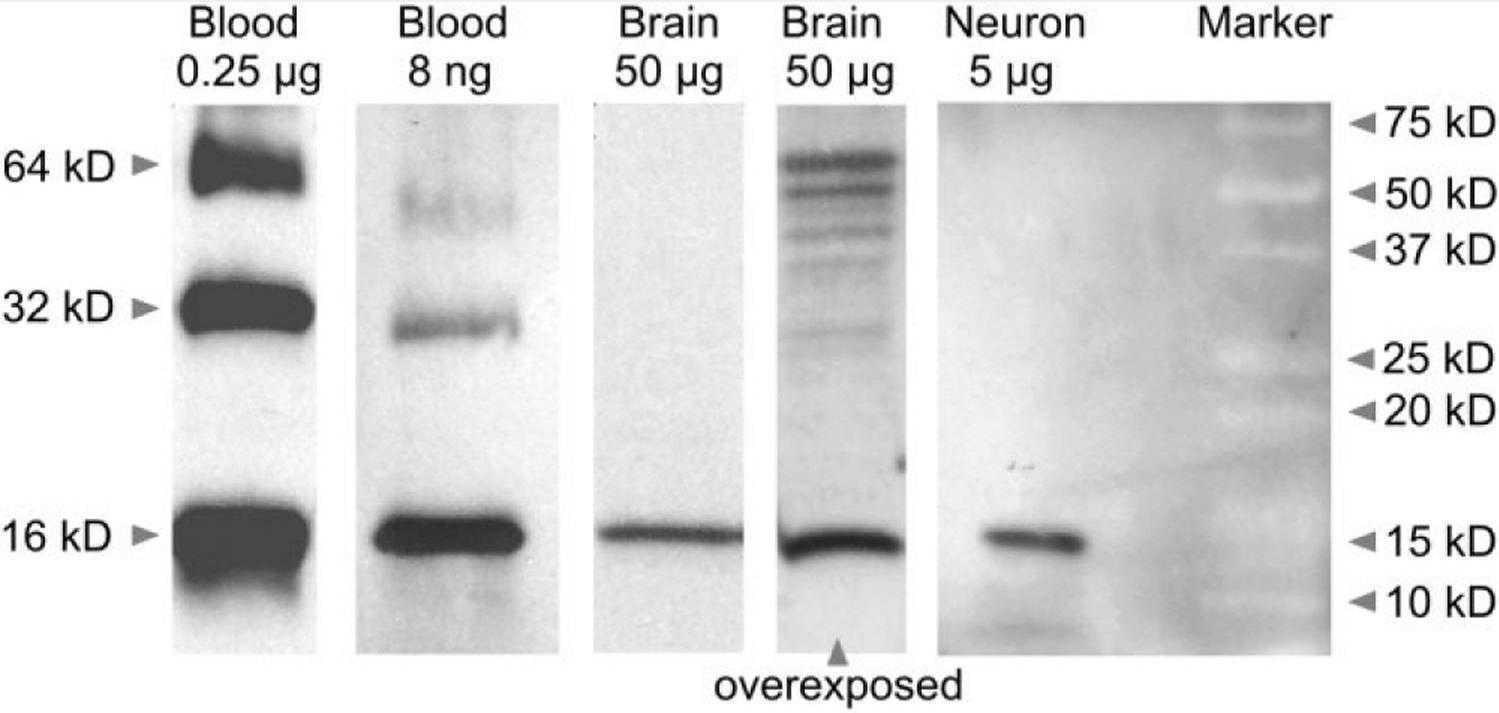
Fig1. Western blot with extracted protein from rat blood (0.25 μg and 8 ng), brain (50 μg), and primary midbrain cultures enriched for neurons ("Neuron" 5 μg) probed with whole hemoglobin antiserum.
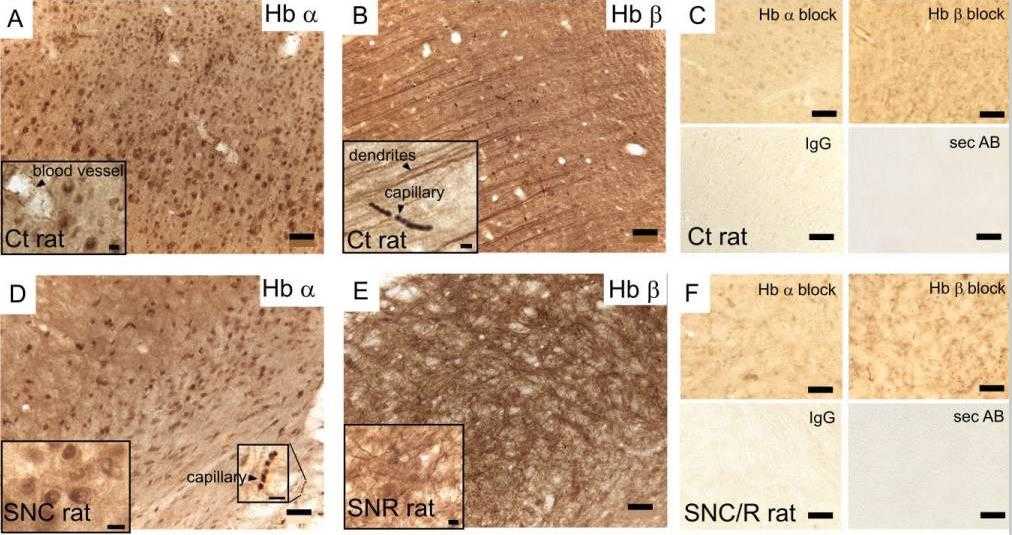
Fig2. Hemoglobin α-or β-chains on 40-μm rat brain sections.
Hemoglobin α2 (also known as hemoglobin A2) is a normally present form of hemoglobin in the human body, which typically makes up about 2-3% of the hemoglobin in adults. Hemoglobin alpha 2 is part of hemoglobin, a key protein in red blood cells that transports oxygen. In some cases, artificial hemoglobin can be used as a substitute for red blood cells to treat conditions such as anemia, shock, and brain obstruction. Hemoglobin α2 can be used as an iron supplement in the healthcare field to treat symptoms of iron deficiency anemia or iron deficiency. In the food industry, hemoglobin α2 can be used as a food-grade colorant and flavoring agent, especially in artificial meat products, to simulate the color and flavor of real meat. In vascularless tissues such as cartilage, hemoglobin α2 may form hemoglobin bodies by liquid-liquid separation, which act as oxygen storage structures, releasing oxygen in the event of hypoxia and maintaining cell survival and tissue development. Through metabolic engineering and synthetic biology strategies, the use of microbial cell factories to synthesize hemoglobin α2 provides a new way for commercial production.
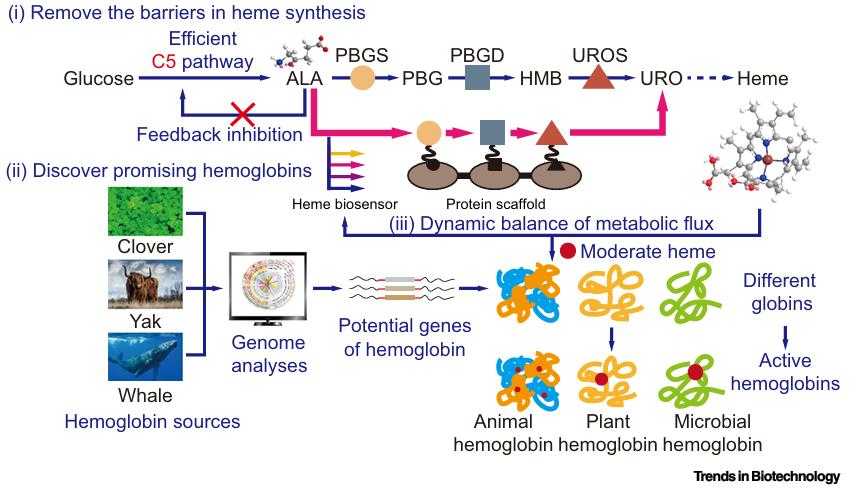
Fig1. Powerful Potential Strategies That Can Be Used to Improve Hemoglobin Biosynthesis in the Future. (Xinrui Zhao, 2021)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All HBA2 Products
Required fields are marked with *
My Review for All HBA2 Products
Required fields are marked with *
Inquiry Basket


