TRIM21
-
Official Full Name
tripartite motif containing 21 -
Overview
This gene encodes a member of the tripartite motif (TRIM) family. The TRIM motif includes three zinc-binding domains, a RING, a B-box type 1 and a B-box type 2, and a coiled-coil region. The encoded protein is part of the RoSSA ribonucleoprotein, which includes a single polypeptide and one of four small RNA molecules. The RoSSA particle localizes to both the cytoplasm and the nucleus. RoSSA interacts with autoantigens in patients with Sjogren syndrome and systemic lupus erythematosus. Alternatively spliced transcript variants for this gene have been described but the full-length nature of only one has been determined. -
Synonyms
TRIM21;tripartite motif containing 21;SSA;RO52;SSA1;RNF81;E3 ubiquitin-protein ligase TRIM21;SS-A;ro(SS-A);52 kDa Ro protein;OTTHUMP00000230775;RING finger protein 81;Sicca syndrome antigen A;tripartite motif-containing 21;sjoegren syndrome type A antigen;tripartite motif-containing protein 21;52 kDa ribonucleoprotein autoantigen Ro/SS-A;Sjogren syndrome antigen A1 (52kDa, ribonucleoprotein autoantigen SS-A/Ro)
Recombinant Proteins
- Human
- Rhesus macaque
- Mouse
- Rat
- Cow
- E.coli
- HEK293
- Wheat Germ
- Sf9 Cells
- Mammalian Cells
- Confidential
- Bovine Thymus
- Insect cells
- In Vitro Cell Free System
- Yeast
- Insect Cells
- His
- DDK
- Myc
- GST
- Non
- T7
- SUMO
- Flag
- Avi
- Fc
Background
What is TRIM21 Protein?
TRIM21 gene (tripartite motif containing 21) is a protein coding gene which situated on the short arm of chromosome 11 at locus 11p15. This gene is part of the TRIM family and its structure features three zinc-binding domains—specifically a RING, B-box type 1, and B-box type 2—along with a coiled-coil region. The protein made from this gene becomes a component of the RoSSA ribonucleoprotein, which teams up with a small RNA molecule. You can find these particles hanging out in both the cytoplasm and the nucleus of cells. Interestingly, RoSSA tends to show up in interactions with autoantigens, especially in patients dealing with conditions like Sjogren syndrome and systemic lupus erythematosus. The TRIM21 protein is consisted of 475 amino acids and TRIM21 molecular weight is approximately 54.2 kDa.
What is the Function of TRIM21 Protein?
TRIM21, part of the tripartite motif (TRIM) family, is a protein known for its RING, B-box, and coiled-coil regions. This protein is a piece of the RoSSA ribonucleoprotein, which hangs out in both the cytoplasm and the nucleus of cells. It's known to interact with certain proteins that are the target of autoimmune reactions in disorders like Sjogren syndrome and lupus. Essentially, TRIM21 is involved in the body's defense mechanism, and sometimes in autoimmune conditions where the body's defense gets a bit confused about what it's attacking. So, its main gig is dealing with immunity on a cellular level, watching for both internal and external threats.
TRIM21 Related Signaling Pathway
TRIM21 is a cool protein that plays a big role in protecting our cells from viruses. Think of it as a cellular watchdog. It's part of what's called the tripartite motif family and has some fancy zinc-binding domains. TRIM21's main gig is to spot and grab onto antibodies that have clung onto viruses inside our cells. Once it clamps on, TRIM21 sends both the virus and the attached antibody to the cell's trash compactor, the proteasome, for destruction. Curiously, TRIM21 itself avoids getting trashed in the process. This self-cleaning operation is crucial in autoimmune diseases like Sjögren's syndrome and lupus, where TRIM21's action can sometimes backfire, making these conditions worse. On the flip side, TRIM21 can be used in labs as a tool to selectively remove certain proteins, helping scientists understand their function or role in cellular processes.
TRIM21 Related Diseases
TRIM21 is like a key player in our immune system's lineup, helping to track down and get rid of unwanted stuff in our cells, like pesky viral proteins. But here's the kicker: while it's supposed to guard us, TRIM21 can sometimes go overboard. It's been linked to autoimmune conditions like systemic lupus erythematosus and Sjögren's syndrome, where it might mistakenly attack the body's own cells, leading to inflammation and damage. So, despite its role as a defender, when TRIM21 overreacts, it can actually spark or worsen autoimmune flare-ups.
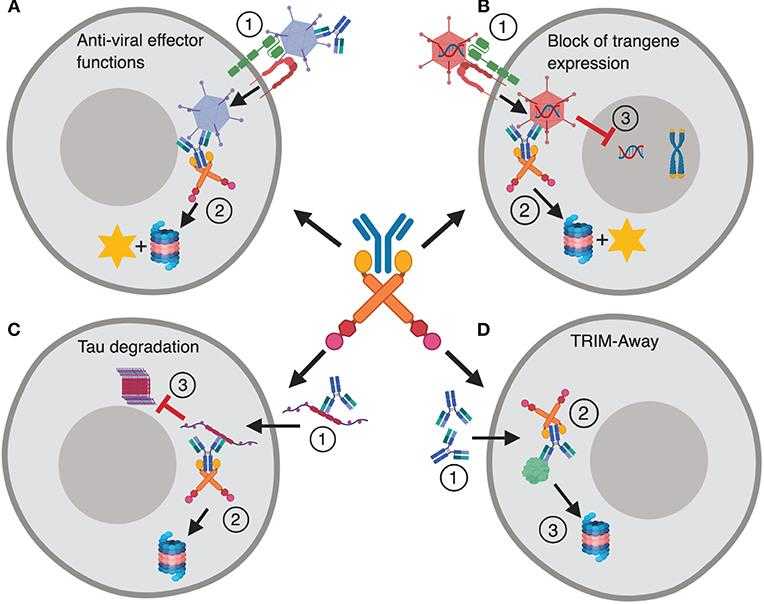
Fig1. TRIM21 in disease and therapy. (Stian Foss, 2019)
Bioapplications of TRIM21
TRIM21 has many potential applications in biomedicine, especially in antiviral therapy and immune research. Its main function is to help cells recognize and remove invading viral proteins, so it is used to design immunotherapies that can clear infections faster. In addition, TRIM21 can also trigger the process of "antibody-mediated protein degradation", which means it can help remove harmful proteins in cells. This feature is very promising in treating some viral infections, autoimmune diseases, and studying the mechanism of protein degradation in cells.
Case Study
Case Study 1: Panrui Lu, 2024
Targeted protein degradation (TPD) is a strategy where special substances, like molecular glues or PROTACs, help break down disease-related proteins by connecting them with E3 ubiquitin ligases. The challenge right now is that we depend on a limited few E3 ligases that are always active. Recently, researchers found that a byproduct of the drug acepromazine, called (S)-ACE-OH, can connect TRIM21, an E3 ubiquitin ligase, with a protein named NUP98. This leads to the destruction of certain nuclear proteins and messes with their usual cell transport roles. By tweaking acepromazine into PROTACs, scientists can target and degrade groups of proteins, like those in complex assemblies, without affecting single proteins. This is because TRIM21 activates when proteins cluster together. Since problems with grouped proteins can lead to issues like autoimmune diseases, brain disorders, and cancer, using TRIM21 as a precise degrader offers a promising way to address these problems directly.
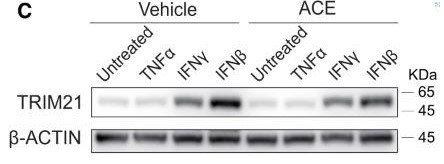
Fig1. Immunoblots of TRIM21 and β-actin in A549 cells.
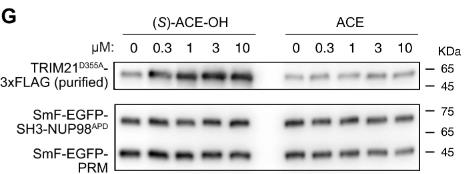
Fig2. Effect of (S)-ACE-OH and ACE on the enrichment of purified TRIM21D355A-3xFLAG protein within SmF-EGFP-PRM/SmF-EGFP-SH3-NUP98APD condensates formed in vitro.
Case Study 2: Wenqing Gao, 2021
Gasdermin-D (GSDMD) is a key player in pyroptosis, a type of cell death triggered when it's cut by inflammatory caspases. This process helps defend the body and react to danger signals. Until now, the only known way to activate GSDMD was through this cleavage. However, research shows that TRIM21 can also positively regulate GSDMD-driven pyroptosis. TRIM21 binds to GSDMD, keeping it stable in resting cells and promoting its active form during pyroptosis. Cells lacking TRIM21 show less cell death when inflammasomes like NLRP3 or NLRC4 are activated. In mice, removing TRIM21 offers protection against inflammation caused by LPS and colitis triggered by dextran sulfate sodium.

Fig3. Co-immunoprecipitation (Co-IP) analysis of GSDMD-Flag with HA-TRIM21 or HA-WWP2 in HEK293T cells.
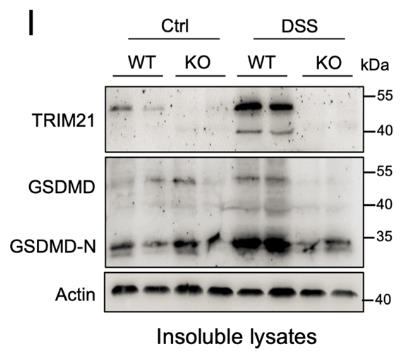
Fig4. GSDMD and TRIM21 protein expression levels in colon tissues were analyzed by immunoblotting.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (TRIM21-543H)
.
.jpg)
Fig2. SDS-PAGE (TRIM21-5540H)
Involved Pathway
TRIM21 involved in several pathways and played different roles in them. We selected most pathways TRIM21 participated on our site, such as Systemic lupus erythematosus, which may be useful for your reference. Also, other proteins which involved in the same pathway with TRIM21 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Systemic lupus erythematosus | HLA-DRB5,HIST2H2AA4,C1QA,HLA-DMA,HIST1H4J,HIST1H4F,HIST1H2AF,HIST1H2AP,C8A,FCGR3 |
Protein Function
TRIM21 has several biochemical functions, for example, DNA binding,RNA binding,identical protein binding. Some of the functions are cooperated with other proteins, some of the functions could acted by TRIM21 itself. We selected most functions TRIM21 had, and list some proteins which have the same functions with TRIM21. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| zinc ion binding | PRICKLE2B,NR6A1B,CA4A,GLO1,NRAP,ZSWIM7,CPA6,LIMA1,PRKCG,PRKCBP1L |
| DNA binding | ZNF684,TDEANC2,MCM2,ZFP639,ELF2B,STRBP,RUNX1,STAT5B,IRF1B,SIX3B |
| protein binding | SYT2,RBL1,NCBP1,FIP1L1,C1S,PDP1,KIF20B,SKP1,VEPH1,DDA1 |
| RNA binding | ZMAT4,RPL14,KHSRP,TUT1,THOC2,HENMT1,SAMD4,PDCD4,RDBP,THOC5 |
| ubiquitin-protein transferase activity | LRSAM1,RNF31,HERC2,MYCBP2,TRAF4B,HECTD1,BIRC5B,FBXW2,RFWD3,BIRC5 |
| identical protein binding | MCM6,TNK2,LTBR,TPD52L1,MSTN,SEPHS1,TYROBP,MCIN,CPT1A,CLDN23 |
| ligase activity | TRAF7,DTX4,UBE3B,MARCH3,RNF149,AMFR,BRCA1,RNF13,PARK2,GLULA |
Interacting Protein
TRIM21 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with TRIM21 here. Most of them are supplied by our site. Hope this information will be useful for your research of TRIM21.
TRIM39;3500;GRAP;UBE2I;TXN2
Resources
Related Services
Related Products
References
- Massie, C; Hudson, M; et al. Absence of an association between anti-Ro antibodies and prolonged QTc interval in systemic sclerosis: A multicenter study of 689 patients. SEMINARS IN ARTHRITIS AND RHEUMATISM 44:338-344(2014).
- Rakebrandt, N; Lentes, S; et al. Antibody- and TRIM21-dependent intracellular restriction of Salmonella enterica. PATHOGENS AND DISEASE 72:131-137(2014).


