Neural Progenitor Cell Markers
Related Symbol Search List
Immunology Background
Background
Neural progenitor cells (NPCs) are a vital component in brain development and regeneration, serving as the precursors for the various cell types that make up the central nervous system. These multipotent cells have the remarkable ability to self-renew and differentiate into neurons, astrocytes, and oligodendrocytes, crucial for the formation of complex neural networks in the brain.
Role in Brain Development
- Neurogenesis
Neural progenitor cells are essential for the generation of neurons during embryonic development. They undergo the process of neurogenesis, where they proliferate and differentiate into neurons that populate different regions of the brain.
- Regional Specification
NPCs are influenced by various signaling pathways, such as the Wnt, Notch, and Sonic Hedgehog pathways, which guide their fate decisions. This ensures the correct formation of brain regions, such as the cerebral cortex, hippocampus, and spinal cord.
- Maintenance of Neural Stem Cell Pool
In the developing brain, NPCs help maintain the balance between self-renewal and differentiation, ensuring a sufficient supply of progenitor cells for later developmental stages.
- Formation of Neurogenic Niches
Throughout development, NPCs contribute to the establishment of neurogenic niches, areas where neurogenesis can occur even in adulthood, such as the subventricular zone (SVZ) and the hippocampus.
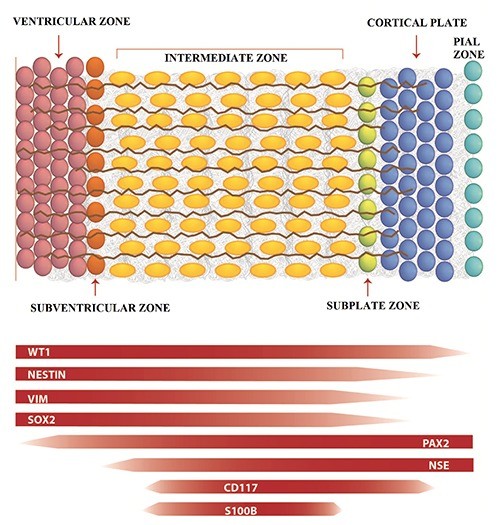 Fig.1 Trend of different expression of markers in different regions of developing cerebral cortex. (Vinci L, et al., 2016)
Fig.1 Trend of different expression of markers in different regions of developing cerebral cortex. (Vinci L, et al., 2016)Role in Brain Regeneration
- Response to Injury
Following brain injury, NPCs can be activated in response to inflammation and damage, migrating to the injury site. They have the potential to differentiate into neurons and glial cells, aiding in tissue repair.
- Neurogenesis in Adults
In certain regions of the adult brain, such as the hippocampus, NPCs continue to produce new neurons throughout life. This is crucial for processes like learning, memory, and mood regulation.
- Potential for Therapeutic Applications
NPCs are being studied for their potential in regenerative medicine. Therapies aiming to harness NPCs could provide avenues for treating neurodegenerative diseases, traumatic brain injuries, and stroke.
- Manipulation and Reprogramming
Advances in techniques such as direct reprogramming allow for the conversion of other cell types into NPCs, potentially offering new strategies for generating neural cells for transplantation and repair.
Neural progenitor cells are fundamental to both brain development and regeneration. Their unique ability to differentiate into multiple cell types and respond to injury underscores their importance in maintaining brain health. Research into NPCs not only enhances our understanding of neurodevelopmental processes but also opens up promising avenues for therapeutic interventions in neurological disorders.
Neural Progenitor Cell Markers
NPCs are characterized by the expression of specific molecular markers that help identify and study these crucial cell populations in the central nervous system. These markers play essential roles in regulating NPC behavior, differentiation, and proliferation. Here is an overview of some key neural progenitor cell markers:
Embryonic Neural Progenitor Cell Markers
- Sox2 (Sex-determining region Y-box 2)
- Nestin
- Pax6 (Paired box gene 6)
Adult Neural Progenitor Cell Markers
- GFAP (Glial Fibrillary Acidic Protein)
- DCX (Doublecortin)
- BLBP (Brain Lipid-Binding Protein)
Neurogenic Niche-Specific Markers
- Ki67
- Notch1
- Mash1 (Ascl1)
Region-Specific Neural Progenitor Cell Markers
- OLIG2
- GLAST (ACSA-1)
Understanding the expression patterns and functions of these neural progenitor cell markers provides valuable insights into NPC biology, neurogenesis, and brain development. These markers serve as essential tools for studying neural progenitor populations in both physiological and pathological conditions, aiding in the development of potential therapeutic strategies for neurological disorders.
Common Neural Progenitor Cell Markers
Here are some common markers used to characterize neural progenitor cells:
| Markers | Details |
|---|---|
| Nestin |
|
| Sox2 |
|
| Neurogenin (Ngn) |
|
| CD133 (Prominin-1) |
|
| Musashi-1 |
|
| Ki67 |
|
| Vimentin |
|
| Pax6 |
|
| BMP4 (Bone Morphogenetic Protein 4) |
|
| Tuj1 (βIII-tubulin) |
|
These markers help in identifying and isolating neural progenitor cells for research and therapeutic purposes. Understanding their expression patterns aids in deciphering the complex processes underlying neural development and regeneration.
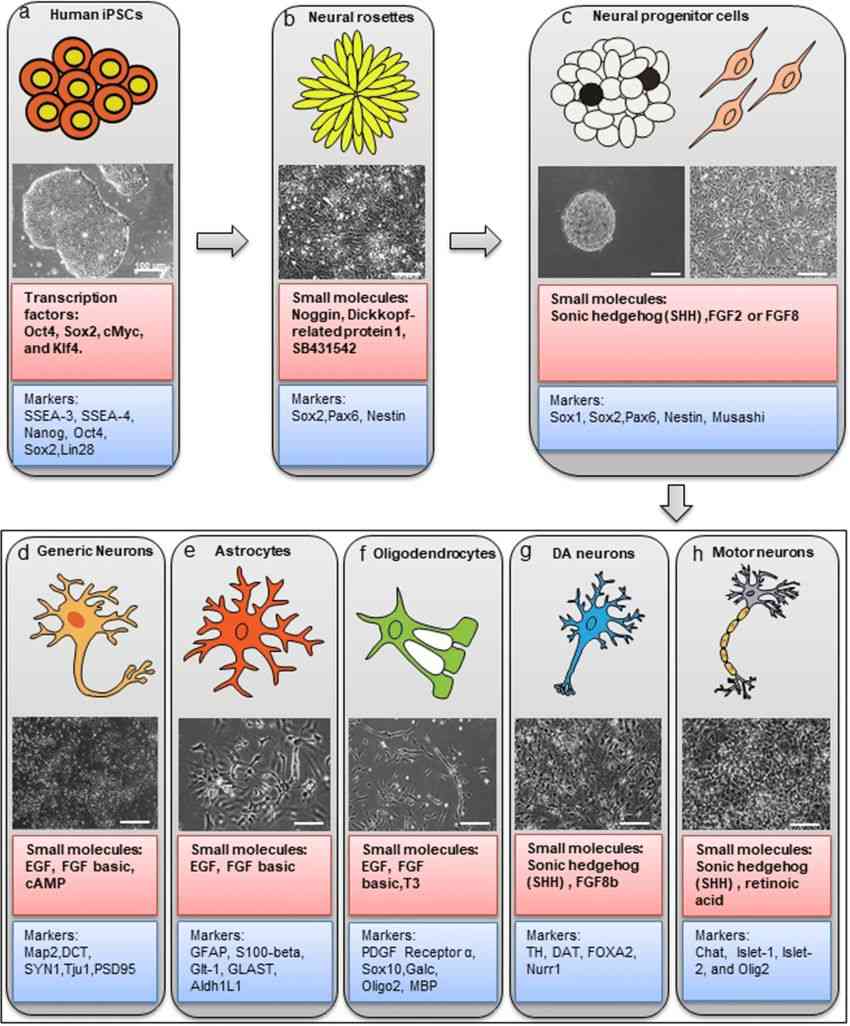 Fig.2 Sequence of cell types of neuronal lineage during NPC generation and neural/glial differentiation with respective differentiation pathways and lineage-specific markers. (Liang X, et al., 2020)
Fig.2 Sequence of cell types of neuronal lineage during NPC generation and neural/glial differentiation with respective differentiation pathways and lineage-specific markers. (Liang X, et al., 2020)Applications of Neural Progenitor Cell Markers
Here are some key applications of neural progenitor cell markers in various fields:
| Fields | Applications | Details |
|---|---|---|
| Research Applications | Identification and Isolation | NPC markers facilitate the identification and isolation of specific cell populations for in-depth study and analysis. |
| Characterization of Cell Populations | By using these markers, researchers can characterize the properties, behavior, and differentiation potential of neural progenitor cells in different contexts. | |
| Understanding Developmental Processes | Studying NPC markers provides insights into the molecular mechanisms underlying neurogenesis, brain development, and cell fate determination. | |
| Disease Modeling | These markers are utilized in disease models to investigate the role of neural progenitor cells in neurological disorders and to develop potential therapeutic strategies. | |
| Drug Screening and Development | NPC markers aid in screening potential drugs and evaluating their effects on neural progenitor cell behavior and differentiation. | |
| Regenerative Medicine Applications | Cell Replacement Therapies | NPC markers guide the selection and manipulation of cells for transplantation in cell replacement therapies for neurodegenerative diseases and brain injuries. |
| Tissue Regeneration | Understanding NPC markers is essential for promoting tissue regeneration and repair in conditions such as stroke, traumatic brain injury, and neurodegenerative disorders. | |
| Induced Pluripotent Stem Cell (iPSC) Technology | NPC markers are utilized in reprogramming techniques to generate patient-specific iPSCs, which can then be differentiated into neural progenitor cells for disease modeling and potential therapies. | |
| Biomarker Development | NPC markers serve as potential biomarkers for assessing disease progression, prognosis, and treatment response in neurological disorders. | |
| Gene Editing and Engineering | These markers are essential for gene editing and engineering approaches to modify neural progenitor cells for therapeutic purposes, such as enhancing their survival, integration, or differentiation potential. | |
| Modeling Brain Disorders | NPC markers facilitate the creation of organoids or other 3D models of the brain, aiding in the study of complex neurological disorders. | |
| Clinical Applications | Patient-Specific Therapies | Using induced pluripotent stem cells (iPSCs) derived from patients, researchers can create patient-specific neural progenitor cells to study individual disease mechanisms and tailor therapies. |
| Biomarkers for Disease | NPC markers may help identify biomarkers for early diagnosis and prognosis of neurodevelopmental and neurodegenerative disorders. |
The applications of neural progenitor cell markers are extensive, spanning basic research to clinical applications in regenerative medicine. These markers not only enhance our understanding of neural development and regeneration but also offer promising avenues for innovative therapies for a range of neurological conditions.
Impact of Neural Progenitor Cell Markers on Development and Disease
Neural progenitor cell (NPC) markers play a crucial role in understanding the development of the nervous system as well as the onset and progression of various diseases. Here's a closer look at their impact:
Developmental Roles
- Cell Fate Determination: Markers such as Sox2, Nestin, and Pax6 are essential for maintaining the multipotency of NPCs and determining their differentiation into neurons, astrocytes, or oligodendrocytes.
- Niche Interactions: NPC markers facilitate interactions with the surrounding microenvironment, influencing cell survival, proliferation, and differentiation.
- Embryonic Development: Proper expression of NPC markers during embryonic development is crucial for forming the neural tube and subsequent structures. Dysregulation can lead to developmental abnormalities.
Neurogenesis
- Regeneration: NPC markers are vital for neurogenesis in the developing and adult brain, impacting processes such as synaptic plasticity and learning.
- Plasticity: The expression of certain markers, like DCX (Doublecortin), can indicate ongoing neurogenesis and neuronal integration in response to environmental stimuli.
Disease Mechanisms
- Cancer: In gliomas and other brain tumors, NPC markers (e.g., SOX2, NESTIN) are often upregulated, suggesting that these tumors may derive from aberrant NPCs. Their presence can imply aggressive behavior and poor prognosis.
- Neurodegenerative Diseases: Altered expression of NPC markers can be observed in diseases like Alzheimer's, Parkinson's, and Huntington's disease, indicating impaired neurogenesis and cell survival.
- Psychiatric Disorders: Changes in NPC populations and marker expression have been linked to conditions such as schizophrenia and depression, highlighting the importance of neurogenesis in mental health.
Therapeutic Implications
- Regenerative Medicine: Understanding NPC markers allows for the development of stem cell therapies aimed at repairing damaged neural tissues.
- Drug Targeting: NPC markers may serve as potential targets for drugs aimed at correcting the aberrant signaling pathways in tumors or neurodegenerative conditions.
Diagnostic Tools
- Biomarkers for Assessment: NPC markers can be used as diagnostic tools to assess the state of neurogenesis in various conditions, providing insights into disease progression and treatment efficacy.
- In summary, neural progenitor cell markers are essential for both healthy development and the pathogenesis of various diseases, making them valuable for research and therapeutic development in neuroscience.
Case Study
Case 1: Rasmussen MA, Hall VJ, Hyttel P. Isolation and culture of porcine neural progenitor cells from embryos and pluripotent stem cells. Methods Mol Biol. 2013;1074:185-198.
The isolation and cultivation of neural progenitor cells (NPCs) from pluripotent stem cells have enabled researchers to conduct in-depth mechanistic investigations of nervous system-related diseases and to discover novel medications in vitro. Furthermore, NPCs are anticipated to assume a pivotal role in forthcoming cell replacement therapies.
The pig has emerged as a vital large animal model, and the establishment of in vitro-derived porcine NPCs offers the opportunity for preclinical safety assessments through transplantation in a porcine biomedical model. This chapter outlines a comprehensive methodology for isolating and cultivating porcine NPCs from porcine embryos or induced pluripotent stem cells. Neural induction is achieved through coculture, and the manual isolation of rosette structures is conducted to ensure a uniform NPC population. By following this protocol, multipotent NPCs can be obtained in approximately one month. These cells exhibit the potential for long-term cultivation and the capacity to differentiate into both neural and glial cells.
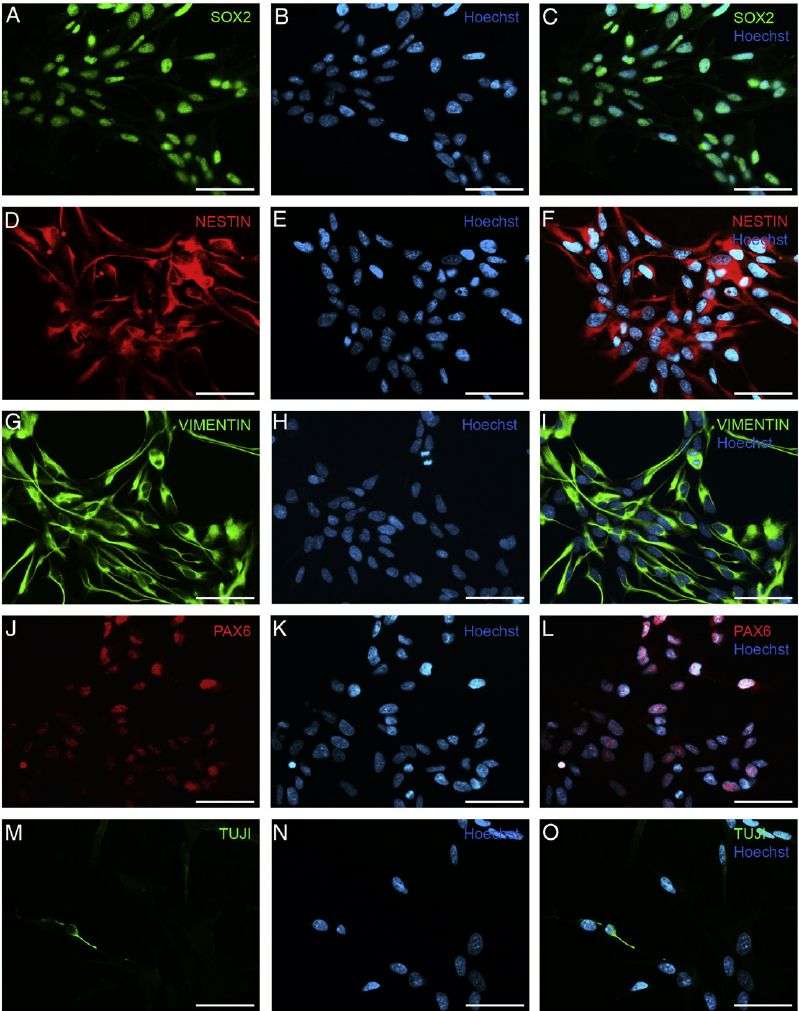 Fig.1 Immunocytochemistry of neural progenitor cells (NPCs).
Fig.1 Immunocytochemistry of neural progenitor cells (NPCs).Case 2: Ryu JK, Cho T, Wang YT, McLarnon JG. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of an inflamed AD brain. J Neuroinflammation. 2009;6:39.
In the study, neural progenitor cells (NPC) were transplanted into rat brains to explore their potential therapeutic benefits for Alzheimer's disease (AD), focusing on reducing inflammation and providing neuroprotection. The NPCs, grown from rat embryo telencephalon tissue, were injected into rat hippocampi previously treated with Abeta1-42 peptide. Results showed that NPC transplantation reduced inflammation (microgliosis) by 38%, lowered proinflammatory cytokine TNF-alpha levels by 40%, and provided neuroprotection, recovering 26% of neurons. The study suggests that NPC transplantation in AD models can mitigate inflammatory responses and offer neuroprotective effects, indicating promise for future therapeutic approaches.
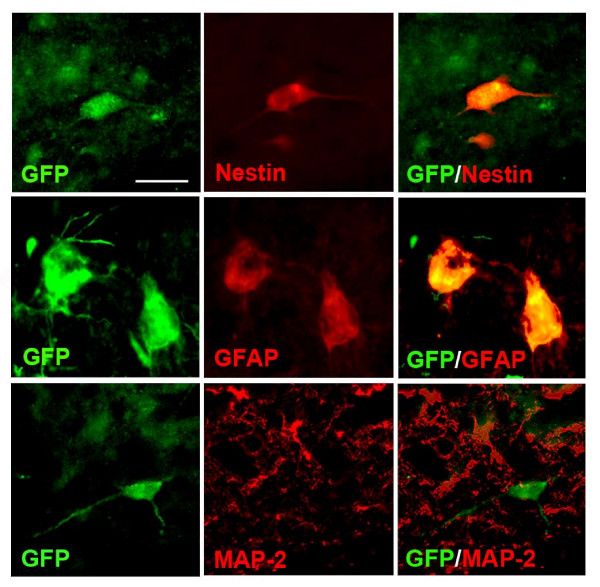 Fig.2 Expression of markers in injected NPC.
Fig.2 Expression of markers in injected NPC.Related References
- Liang X, Kristiansen CK, Vatne GH, Hong Y, Bindoff LA. Patient-specific neural progenitor cells derived from induced pluripotent stem cells offer a promise of good models for mitochondrial disease. Cell Tissue Res. 2020;380(1):15-30.
- Vinci L, Ravarino A, Fanos V, et al. Immunohistochemical markers of neural progenitor cells in the early embryonic human cerebral cortex. Eur J Histochem. 2016;60(1):2563.
- van Strien ME, Sluijs JA, Reynolds BA, Steindler DA, Aronica E, Hol EM. Isolation of neural progenitor cells from the human adult subventricular zone based on expression of the cell surface marker CD271. Stem Cells Transl Med. 2014;3(4):470-480.
