Neural Crest Cell Markers
Related Symbol Search List
Immunology Background
Background
Neural crest cells (NCCs) are a unique population of multipotent cells that arise during the early stages of embryonic development. They play a critical role in the formation of various tissues and structures in the body, particularly in vertebrates. Their ability to migrate and differentiate into diverse cell types makes them essential for many developmental processes.
Overview of Neural Crest Cell Development
| Origin | Neural crest cells originate from the ectoderm, which is one of the three primary germ layers formed during gastrulation.
Specifically, NCCs emerge from the neuroectoderm, migrating away from the developing neural tube, which ultimately gives rise to the central nervous system. |
| Formation | Neural crest formation begins with the upregulation of specific genes (e.g., SNAIL, SLUG, SOX10) that promote epithelial-to-mesenchymal transition (EMT), allowing NCCs to detach from the neuroectoderm. |
Role in Embryonic Development
Neural crest cells are involved in numerous developmental processes, including:
| Migration | After their specification, NCCs undergo extensive migration throughout the embryo. They travel to various regions, giving rise to different structures depending on their destination. |
| Differentiation |
NCCs can differentiate into a wide array of cell types, including:
|
| Contributions to Structures |
Neural crest cells play a vital role in forming structures such as:
|
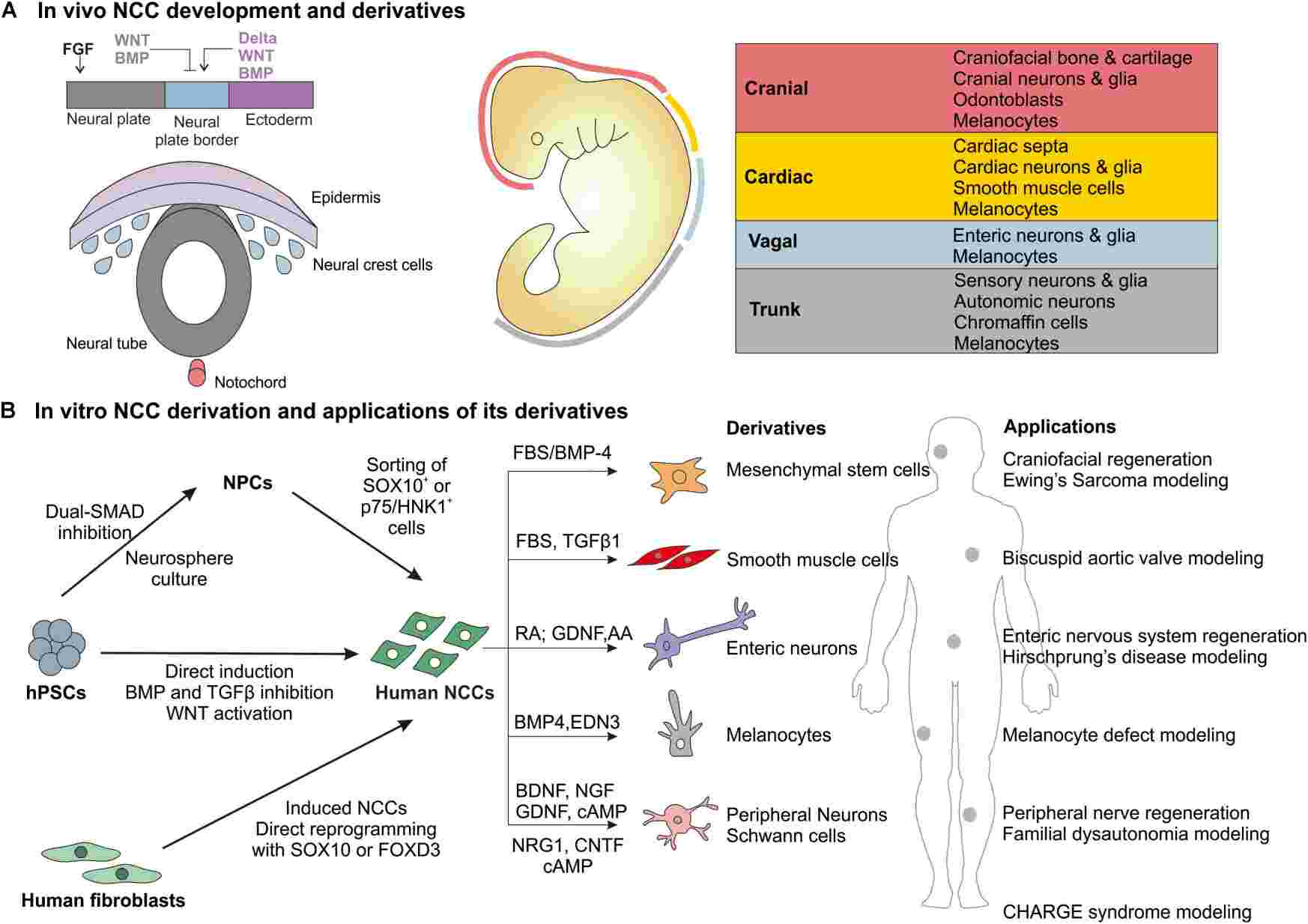 Fig.1 Overview of in vivo NCC development and derivatives and in vitro derivation of human NCCs and major applications. (Srinivasan A, et al., 2019)
Fig.1 Overview of in vivo NCC development and derivatives and in vitro derivation of human NCCs and major applications. (Srinivasan A, et al., 2019)Neural Crest Cell Markers
Neural crest cell markers are essential tools for researchers to identify, track, and study these multipotent cells during embryogenesis. These markers help in understanding the diverse fates and functions of neural crest cells as they differentiate into various cell types in the developing embryo.
Common Neural Crest Cell Markers
Commonly used markers for identifying and studying neural crest cells include a range of proteins, transcription factors, and cell surface antigens. These markers help researchers track the development, migration, and differentiation of neural crest cells. Here are some common neural crest cell markers:
| Type | Details |
|---|---|
| Transcription Factors |
|
| Cell Surface Markers |
|
| Signal Transduction Molecules |
|
| Other Markers |
|
| Phenotypic Markers |
|
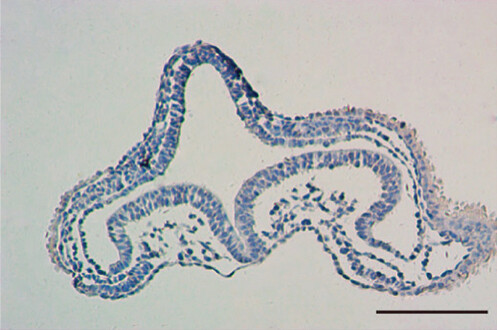 Fig.2 The migratory pathway and pattern of NCSCs were observed with Sox10 as a marker. (Yang LN, et al., 2020)
Fig.2 The migratory pathway and pattern of NCSCs were observed with Sox10 as a marker. (Yang LN, et al., 2020)Research Applications of Neural Crest Cell Markers
Neural crest cell markers serve as invaluable tools in various research applications, enabling scientists to study neural crest cell differentiation, migration, and lineage specification. These markers help researchers track the behavior of neural crest cells throughout development and in disease contexts. Here is a discussion of the diverse research applications of neural crest cell markers:
| Research Applications | Details |
|---|---|
| Neural Crest Cell Differentiation |
|
| Neural Crest Cell Migration |
|
| Lineage Specification and Fate Determination |
|
| Disease Modeling and Drug Development |
|
| Regenerative Medicine and Tissue Engineering |
|
| Developmental Biology and Evolutionary Studies |
|
In conclusion, neural crest cell markers play a critical role in advancing our understanding of neural crest cell biology, development, and disease. By leveraging these markers in various research applications, scientists can unravel the complexities of neural crest cell behavior and pave the way for new discoveries in developmental biology, regenerative medicine, and therapeutic interventions.
Impact of Neural Crest Cell Markers on Development and Disease
Neural crest cell markers have a significant impact on both normal development and disease pathology. These markers help in identifying, tracking, and understanding the behavior of neural crest cells, which are crucial for the formation of various tissues and organs in vertebrate embryos. Here is an exploration of the impact of neural crest cell markers on development and disease, along with relevant examples:
| Type 1 | Type 2 | Marker | Details |
|---|---|---|---|
| Impact on Development | Fate Determination and Differentiation | Sox10
p75NTR and Twist |
Sox10 is essential for the development and maintenance of neural crest cells. Its expression is critical for the differentiation of neural crest cells into various cell types, including neurons, glial cells, and melanocytes.
NCCs contribute to the formation of facial structures and skull bones. p75NTR and Twist are critical in craniofacial development. Mutations in these markers can cause Crouzon syndrome or Treacher Collins syndrome, both characterized by abnormal craniofacial morphology. |
| Migration and Tissue Patterning |
HNK-1 (CD57) Snail |
HNK-1 is a cell surface antigen expressed in migrating neural crest cells. Its presence helps in guiding neural crest cell migration and contributes to the proper patterning of tissues derived from neural crest cells. NCCs migrate to different parts of the embryo and differentiate into various cell types, including neurons, glial cells, and melanocytes. HNK-1 and Snail are commonly used to identify migrating neural crest cells. Defects in NCC migration can lead to congenital conditions like Hirschsprung disease, where neural crest-derived enteric ganglia fail to populate the distal colon. |
|
| Lineage Specification | Tyrosine hydroxylase | Tyrosine hydroxylase is expressed in catecholaminergic neural crest derivatives, such as sympathetic neurons. Its identification helps in tracing the lineage and fate of neural crest cells giving rise to specific cell types. | |
| Impact on Disease | Neurocristopathies | AP-2α (TFAP2A) | Mutations in TFAP2A have been associated with Branchio-Oculo-Facial Syndrome, a neurocristopathy affecting structures derived from the neural crest, including craniofacial features and ear anomalies. |
| Neural Crest Cell - derived Cancers | Melan-A (MART-1) | Melan-A is a specific marker for melanocytes derived from the neural crest. Dysregulation of melanocyte markers like Melan-A is observed in melanoma, a type of skin cancer arising from melanocytes. | |
| Neurodevelopmental Disorders |
Nestin PHOX2B and N-MYC |
Nestin, expressed in neural crest cells during early development, is associated with neurodevelopmental disorders like neurofibromatosis, where mutations in neural crest cell markers contribute to the formation of tumors along nerves. Aberrant NCC behavior can lead to neuroblastoma and other tumors. PHOX2B and N-MYC are associated with neuroblastoma. Overexpression of these markers can indicate aggressive tumor behavior. |
Neural crest cell markers are vital in both understanding normal developmental processes and identifying mechanisms underlying various diseases. Their study continues to provide insights into congenital disorders, cancer, and potential regenerative therapies.
Case Study
Case 1: Betters E, Charney RM, Garcia-Castro MI. Early specification and development of rabbit neural crest cells. Dev Biol. 2019 Dec 15;456(2):234.
The remarkable migratory and differentiation abilities of neural crest cells are well-known in various model organisms. While early neural crest development has been extensively studied in non-mammalian models like Xenopus and Aves, investigations into the early specification of neural crest cells in mammalian embryos, particularly using murine models, are limited. To enhance our understanding of mammalian neural crest formation and for comparative studies in humans, researchers have turned to the rabbit as a mammalian system. By analyzing the expression patterns of established neural crest markers in rabbit embryos from early gastrula to later neurula stages and comparing them with migratory neural crest markers in chick embryos, this study sheds light on early neural crest specification in rabbits. The research reveals the presence of a specified neural crest cell population in rabbit gastrula even before the visible expression of neural crest markers. Moreover, through explant assays, the study highlights the essential role of FGF signaling in early rabbit neural crest development, as demonstrated by the repression of neural crest marker expression upon SU5402 treatment. This pioneering study establishes the rabbit as a valuable model for investigating neural crest development and provides crucial insights into mammalian neural crest specification and the significance of FGF signaling in this process.
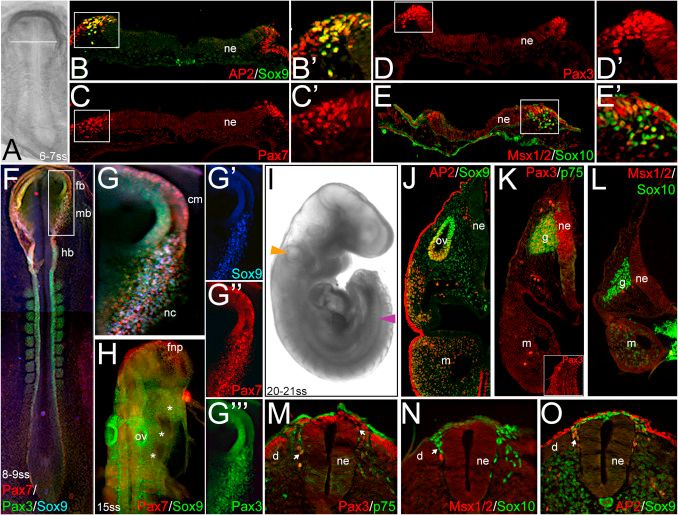 Fig.1 Markers of pre-migratory and migratory rabbit neural crest cells.
Fig.1 Markers of pre-migratory and migratory rabbit neural crest cells.Case 2: Hari L, Miescher I, Shakhova O, et al. Temporal control of neural crest lineage generation by Wnt/β-catenin signaling. Development. 2012;139(12):2107-2117.
The control of various aspects of neural crest development, including induction, lineage determination, and differentiation, is orchestrated by Wnt/β-catenin signaling. In mice, manipulating β-catenin levels in premigratory neural crest cells disrupts the formation of sensory neurons and melanocytes. Interestingly, activating β-catenin in these cells inhibits melanocyte generation while promoting sensory neurogenesis. This prompts an inquiry into how Wnt/β-catenin signaling influences the emergence of distinct neural crest lineages.
By selectively activating β-catenin at different developmental stages using various Cre lines, researchers reveal that Wnt/β-catenin signaling temporally governs neural crest cell fate decisions in vivo. Activation in migratory neural crest cells induces ectopic melanoblasts while repressing other lineages. These melanoblasts arise in diverse locations, indicating a broad potential for neural crest cell differentiation. Notably, the responsiveness of neural crest cells to Wnt/β-catenin signaling is time-constrained during migration, with a limited window for influencing cell fate. This study underscores the multipotency of neural crest cells both before and after leaving the neural tube, highlighting their ability to adapt to external signals in a temporally regulated manner.
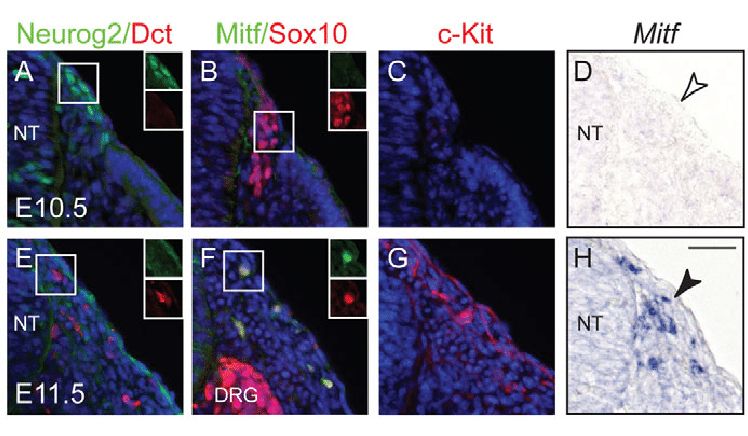 Fig.2 Sequential expression of sensory and melanocyte markers in neural crest cells.
Fig.2 Sequential expression of sensory and melanocyte markers in neural crest cells.Case 3: Jäger K, Larribère L, Wu H, Weiss C, Gebhardt C, Utikal J. Expression of neural crest markers GLDC and ERRFI1 is correlated with melanoma prognosis. Cancers. 2019; 11(1):76.
During the progression of malignant melanoma, the regulation of specific genes observed in neural crest cell formation becomes relevant. Developing neural crest models is crucial for identifying potential biomarkers for melanoma prognosis. Using a human induced Pluripotent Stem Cells (iPSC) approach, novel neural crest-associated genes with increased expression in melanoma were identified. Eight candidate genes, including CD271, GLDC, and ERRFI1, were tested for prognostic value in advanced melanoma and high-risk primary melanoma samples. GLDC and TWIST exhibited significantly higher expression in patients with early metastasis or poor survival outcomes. Specifically, GLDC protein expression showed a notable association with melanoma prognosis in this iPSC-based study.
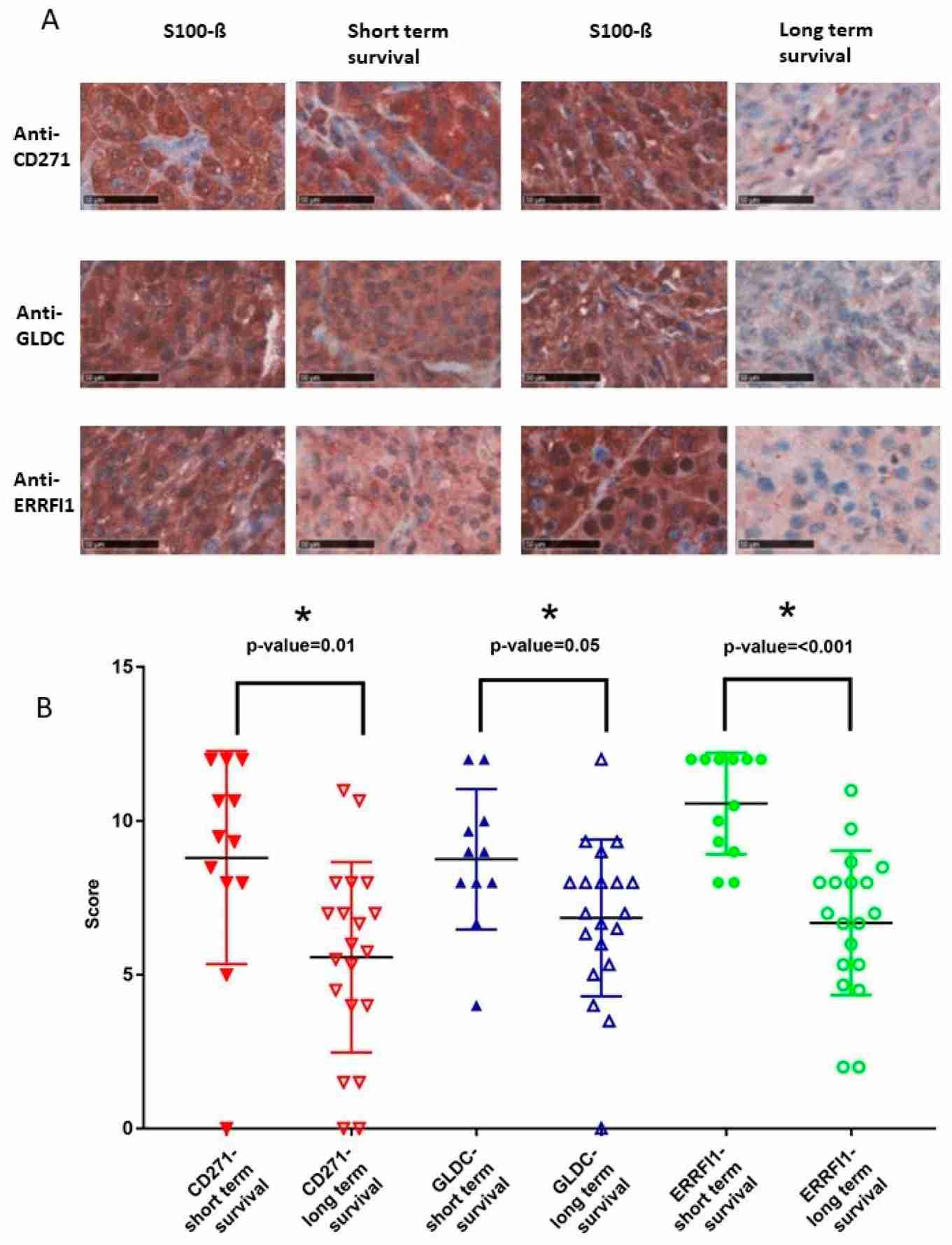 Fig.3 Neural crest markers CD271, GLDC, and ERRFI1 are highly expressed in advanced melanoma patients with bad prognosis.
Fig.3 Neural crest markers CD271, GLDC, and ERRFI1 are highly expressed in advanced melanoma patients with bad prognosis.Related References
- Srinivasan A, Toh YC. Human pluripotent stem cell-derived neural crest cells for tissue regeneration and disease modeling. Front Mol Neurosci. 2019;12:39.
- Achilleos, A., Trainor, P. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res 22, 288–304 (2012).
- Soto J, Ding X, Wang A, Li S. Neural crest-like stem cells for tissue regeneration. Stem Cells Transl Med. 2021;10(5):681-693.
- Yang LN, Huang WK, Li XL, Bai YZ, Zhang SC. Sox10 is a specific biomarker for neural crest stem cells in immunohistochemical staining in wistar rats. Dis Markers. 2020;2020:8893703.
