Mediators in B Cell Development
Related Symbol Search List
Immunology Background
Background
B cell development is a complex process that occurs primarily in the bone marrow and involves several stages of maturation. The development of B cells is tightly regulated by various mediators, including growth factors, cytokines, chemokines, and cell adhesion molecules. Here is an overview of B cell development and the role of these mediators:
- Hematopoietic Stem Cell (HSC) Commitment: B cell development begins with the commitment of hematopoietic stem cells to the lymphoid lineage. This commitment is influenced by various growth factors, such as stem cell factor (SCF), Flt3 ligand, and interleukin-7 (IL-7). These growth factors promote the survival and proliferation of lymphoid progenitor cells.
- Pro-B Cell Stage: Lymphoid progenitor cells undergo further differentiation into pro-B cells. At this stage, IL-7 plays a critical role in promoting the survival and proliferation of pro-B cells. IL-7 receptor signaling activates several key transcription factors, such as E2A and EBF1, which are essential for B cell lineage commitment and progression.
- Pre-B Cell Stage: Pro-B cells undergo rearrangement of immunoglobulin heavy chain (IgH) genes to generate functional heavy chain variable regions. Successful IgH rearrangement leads to the expression of pre-B cell receptors (pre-BCR) on the cell surface. The pre-BCR consists of a heavy chain paired with surrogate light chains. The pre-BCR signaling, mediated by components like Igα and Igβ, is critical for pre-B cell survival, proliferation, and differentiation.
- Immature B Cell Stage: Pre-B cells that successfully pass the pre-B cell checkpoint differentiate into immature B cells. At this stage, the expression of functional surface immunoglobulin (sIg) composed of both IgH and IgL chains occurs. The sIg serves as the B cell receptor (BCR) for antigen recognition. The BCR signaling is crucial for the survival, maturation, and selection of immature B cells.
- B Cell Maturation and Selection: Immature B cells migrate from the bone marrow to secondary lymphoid organs, such as the spleen and lymph nodes, to complete their maturation. During this process, B cells undergo positive and negative selection. Positive selection occurs when immature B cells encounter and bind to self-antigens in the lymphoid organs, leading to their survival and maturation. Negative selection eliminates immature B cells that strongly react to self-antigens to prevent the development of autoimmunity.
Throughout B cell development, various mediators play essential roles in regulating different stages of maturation. These mediators include growth factors, cytokines, chemokines, cell adhesion molecules, and others.
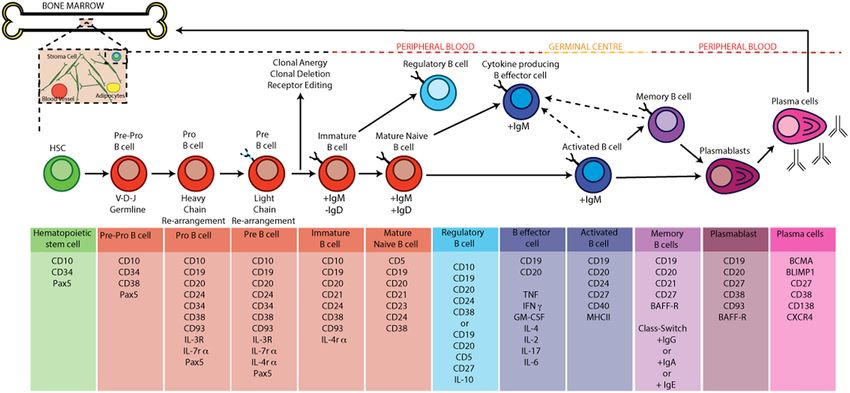 Fig.1 B cell development and activation in humans. (Wilkinson MGL, et al., 2019)
Fig.1 B cell development and activation in humans. (Wilkinson MGL, et al., 2019)Mediators in B Cell Development
B cell development is a highly regulated process involving the sequential differentiation and maturation of B cell progenitors. The interplay between growth factors, cytokines, chemokines, cell adhesion molecules, and other key mediators is crucial for the proper progression, survival, migration, and selection of B cells at different stages of development.
| Types | Functions |
|---|---|
| Key Mediators |
|
| Growth Factors |
|
| Chemokines |
|
| Cytokines |
|
| Cell Adhesion Molecules |
|
These mediators, including cytokines, chemokines, growth factors, and specific molecules, contribute to the regulation of B cell development, survival, differentiation, migration, and signaling processes.
Disordered Effects of Mediators on B Cell Development and the Immune Response
Dysregulation of mediators involved in B cell development can have significant impacts on B cell development and the immune response. Here are some effects of dysregulation:
| Impacts | Details |
|---|---|
| Impaired B Cell Development | Dysregulation of growth factors, cytokines, and chemokines can disrupt the normal progression of B cell development. For example, deficiencies in IL-7 or its receptor can result in impaired expansion and survival of pro-B and pre-B cells, leading to a reduced pool of mature B cells. This can result in immunodeficiency and increased susceptibility to infections. |
| Altered B Cell Repertoire | Dysregulation of mediators can influence the diversity and specificity of the B cell repertoire. Proper B cell development involves the generation of a diverse repertoire of B cells expressing different immunoglobulin specificities. Dysregulation can lead to skewed or restricted B cell repertoires, reducing the ability to mount effective immune responses against a wide range of pathogens. |
| Aberrant B Cell Activation and Differentiation | Dysregulated mediators can disrupt the normal processes of B cell activation and differentiation. For instance, aberrant BCR signaling resulting from dysregulation of signaling molecules or co-receptors can lead to excessive or inadequate B cell activation. This can contribute to autoimmune disorders or impaired immune responses to infections. |
| Altered Antibody Production | Dysregulation of cytokines involved in B cell differentiation and class switching, such as IL-4 and IL-6, can affect the production and class switching of antibodies. Imbalances in cytokine signaling can lead to altered antibody production, including reduced antibody levels or the production of antibodies with altered isotypes or affinities. This can impact the effectiveness of humoral immune responses. |
| Autoimmunity and Allergic Responses | Dysregulation of B cell development and immune responses can contribute to the development of autoimmune diseases and allergic reactions. Dysregulated B cell activation and differentiation can lead to the production of autoantibodies, which recognize and attack self-tissues, causing autoimmune disorders. In the case of allergies, dysregulated B cell responses can result in the production of excessive amounts of IgE antibodies, leading to hypersensitivity reactions. |
| Impaired Immune Response to Pathogens | Dysregulated B cell development and function can compromise the immune response to pathogens. Reduced B cell numbers, altered B cell repertoires, or impaired antibody production can result in decreased ability to neutralize pathogens, leading to increased susceptibility to infections. |
Overall, dysregulation of mediators involved in B cell development can disrupt normal B cell maturation, activation, differentiation, and antibody production. This can have implications for the immune response, leading to immunodeficiency, autoimmune diseases, allergies, and impaired defense against pathogens.
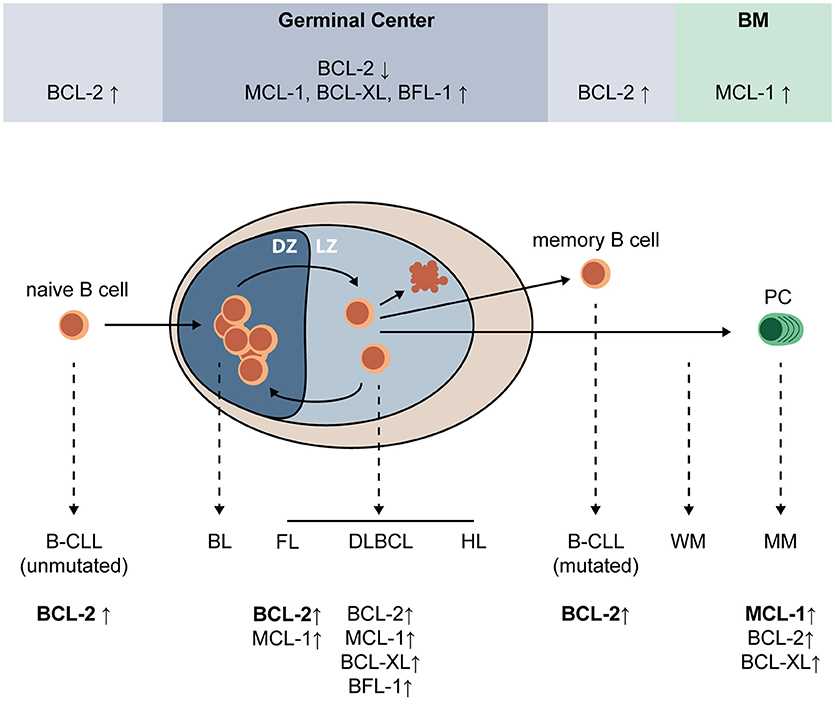 Fig.2 Expression of pro-survival BCL-2 family proteins during plasma cells (PC) differentiation and after malignant transformation of (post-) GC B-cells. (Slomp A, et al., 2018)
Fig.2 Expression of pro-survival BCL-2 family proteins during plasma cells (PC) differentiation and after malignant transformation of (post-) GC B-cells. (Slomp A, et al., 2018)Current Research on Mediators in B Cell Development and Their Potential Therapeutic Implications
Current research on mediators in B cell development is focused on understanding the underlying mechanisms and exploring their therapeutic implications. Here are some areas of research and potential therapeutic implications:
Cytokines and Growth Factors
Researchers are investigating the roles of cytokines and growth factors, such as IL-7, IL-4, and SCF, in B cell development and how their dysregulation contributes to immune disorders. Targeting these mediators or their receptors could potentially modulate B cell development and function in diseases like immunodeficiencies, autoimmune disorders, and lymphoid malignancies.
B Cell Receptor Signaling
Dysregulated B cell receptor (BCR) signaling is implicated in autoimmune diseases and lymphoid malignancies. Researchers are studying the signaling pathways downstream of the BCR and identifying potential targets for therapeutic intervention. Modulating BCR signaling could help restore immune tolerance or inhibit aberrant B cell activation and proliferation.
Transcription Factors
Transcription factors play critical roles in B cell development and differentiation. Current research focuses on understanding the functions of transcription factors like E2A, EBF1, and Pax5 and their interactions with other regulators. Manipulating these transcription factors could have therapeutic implications for modulating B cell development and targeting lymphoid malignancies.
Chemokine Receptors
Chemokine receptors and their ligands are involved in B cell migration, localization, and homing to lymphoid organs. Targeting specific chemokine receptors could potentially modulate B cell trafficking and migration, impacting immune responses. This research may have implications for developing therapies targeting lymphoid malignancies or autoimmune diseases by manipulating B cell localization and homing.
Immunomodulatory Therapies
Understanding the mediators involved in B cell development has led to the development of immunomodulatory therapies targeting B cells. For example, monoclonal antibodies that selectively target B cells, such as anti-CD20 antibodies (e.g., rituximab), have been used in the treatment of autoimmune diseases and B cell malignancies.
Gene Editing and Cell Therapies
Advances in gene editing technologies, such as CRISPR-Cas9, offer potential therapeutic applications for manipulating mediators involved in B cell development. Precise editing of genes involved in B cell development could correct genetic defects causing immunodeficiencies or enhance immune responses against pathogens or tumors. Additionally, cell-based therapies, such as chimeric antigen receptor (CAR) T cell therapy, are being explored to target B cell malignancies by redirecting T cells to recognize and eliminate B cells expressing specific antigens.
Ongoing research in these areas aims to deepen our understanding of B cell development and identify potential therapeutic targets for immune-related disorders, immunodeficiencies, autoimmune diseases, and lymphoid malignancies. These advances have the potential to lead to the development of more targeted and personalized treatments in the future.
Case Study
Case 1: Cai Z, Zhang L, Cao M, et al. Generation of a Murine Model for c-MYC and BCL2 Co-expression B Cell Lymphomas. Front Oncol. 2020;10:1007.
c-MYC and BCL2 play important roles in normal B-cell differentiation and tumorigenesis by affecting different cellular processes (apoptosis, proliferation, cell-cycle control, growth, cell migration, and metabolism). Diffuse large B-cell lymphoma (DLBCL) are phenotypically and genetically heterogeneous.
The data indicated that c-MYC and BCL2 Co-expression in GC B cells could induce B cell lymphoma. The authors explored whether these tumor cells could metastasize to the other organs. The authors analyzed the B cells in the peripheral blood of AID+ ki/+ mice and WT littermate controls, and observed increased B220+ B cells and B220+Fas+ B cells in the AID+ ki/+ mice. But the proportion of GC B cells did not increase significantly (A, B). Furthermore, a gradual increase of B220+ B cells and B220+Fas+ B cells were observed from 6 to 10 weeks (C, D).
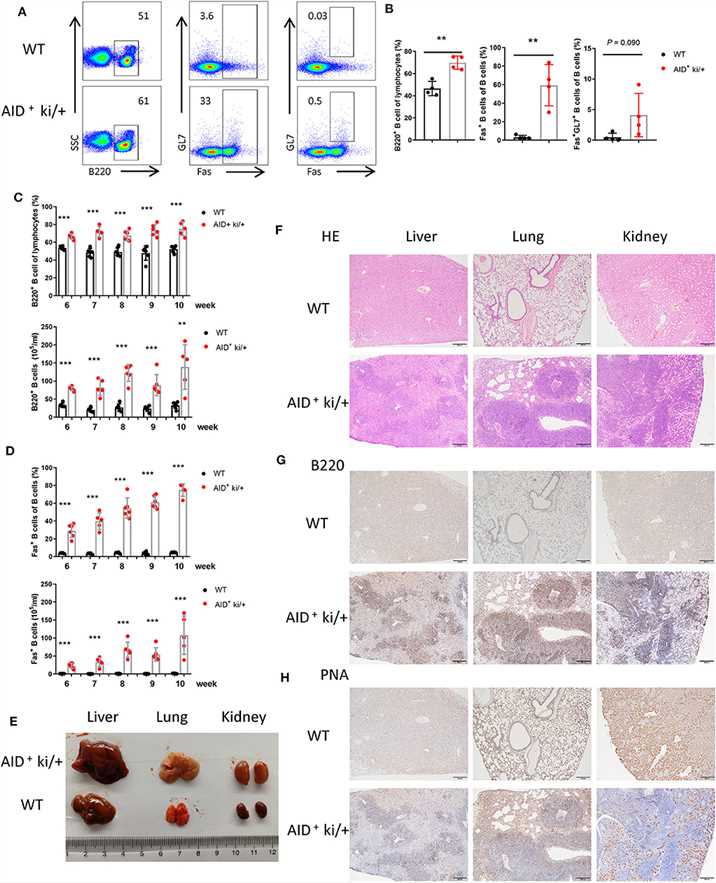 Fig.1 B cell expansion in the lung, liver, and kidney from blood in GC B cell specific c-MYC and BCL2 expression mice.
Fig.1 B cell expansion in the lung, liver, and kidney from blood in GC B cell specific c-MYC and BCL2 expression mice.Case 2: Tessoulin B, Papin A, Gomez-Bougie P, et al. BCL2-Family Dysregulation in B-Cell Malignancies: From Gene Expression Regulation to a Targeted Therapy Biomarker. Front Oncol. 2019;8:645.
To gain insight into BCL2-family expression and regulation across most frequent mature B cell malignancies, the authors analyzed the BCL2-family expression in ten different hematological disorders i.e., MCL, BL, DLBCL, FL, B-cell prolymphocytic leukemia (BPLL), CLL, HCL, mucosa-associated lymphoid tissue (MALT), SMZL, MM, through normalization of Affymetrix Human Genome U133 Plus 2.0 public datasets. The authors analyzed the common modulations across all B-cell neoplasms in comparison with their respective normal counterpart.
In contrast to most mature B cell malignancies, MM and BL did not show major modulations of anti-apoptotic genes when compared to their normal counterparts (A). Pro-apoptotic BH3-only have a tendency to be downregulated in all mature B cell malignancies compared to their relative normal control, BCL2L11, coding for Bim protein, being the most frequently significantly deregulated gene (B). Regarding pro-apoptotic effectors the authors observed a BAX/BAK1 switch of expression in malignant B cells compared to their normal counterparts. Indeed, whereas BAX was elevated, BAK1 appeared downregulated in all malignancies, excepted in MM and BL (A).
To compare the 10 entities studied in regard to their BCL2-family profile, the authors performed a Principal Component Analysis (PCA, C). The authors observed that CLL and MM displayed unique profiles. The variable plot highlighted that CLL profile was mostly carried by the expression of BCL2, BMF, PMAIP1, coding for Noxa protein, and the absence of BID whereas MM cells were characterized by the projection of BCL2L1, BAK1, and BCL2L11 and the absence of BCL2A1 (C).
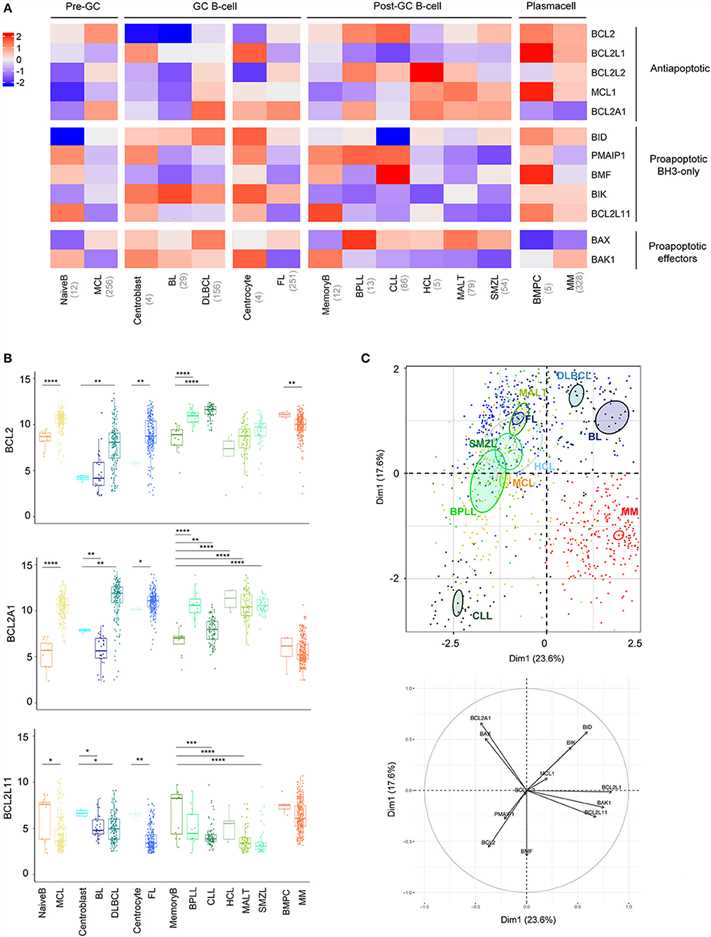 Fig.2 BCL2-family is strongly deregulated in the landscape of B-cell malignancies.
Fig.2 BCL2-family is strongly deregulated in the landscape of B-cell malignancies.References
- Wilkinson MGL, Rosser EC. B Cells as a Therapeutic Target in Paediatric Rheumatic Disease. Front Immunol. 2019;10:214.
- Slomp A, Peperzak V. Role and Regulation of Pro-survival BCL-2 Proteins in Multiple Myeloma. Front Oncol. 2018;8:533.
