Recombinant Renilla Luciferase Protein
| Cat.No. : | Luciferase-01R |
| Product Overview : | Recombinant Full- length Renilla reniformis Luciferase protein without tag was expressed in E.coli. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Renilla reniformis |
| Source : | E.coli |
| Tag : | Non |
| Description : | The Green Renilla luciferase is a 36kDa protein produced by a derivative of the wild type Renilla luciferase gene from the sea pansy, Renilla reniformis. Compared to the wild type luciferase, Green Renilla is more stable in serum and has an the emission spectrum that is shifted toward the green region. The protein provides extremely bright flash signal that decays rapidly. |
| Form : | Liquid |
| Molecular Mass : | 30 kDa |
| Purity : | > 95% |
| Notes : | This product is furnished for LABORATORY RESEARCH USE ONLY. Not for diagnostic or therapeutic use. |
| Storage : | Upon arrival, the protein may be stored for 2 weeks at 4 centigrade. For long term storage, it is recommended to store at -20 centigrade or -80 centigrade in appropriate aliquots. Avoid repeated freeze-thaw cycles. |
| Storage Buffer : | Supplied in 1x SDS Loading Buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.002% bromphenol blue) at the concentration of 20 ng/uL. |
| Synonyms | Renilla luciferin 2 monooxygenase; Renilla type luciferase |
| ◆ Recombinant Proteins | ||
| Luciferase-10F | Recombinant Firefly Luciferase, Thermostable | +Inquiry |
| Luciferase-105G | Active Recombinant Gaussia Luciferase Protein, Streptavidin-Labled | +Inquiry |
| Luciferase-10R | Active Recombinant Renilla reniformis Luciferase (1-311), N-His-tagged | +Inquiry |
| Luciferase-104G | Active Recombinant Gaussia Luciferase Protein | +Inquiry |
| Luciferase-09R | Recombinant Renilla Luciferase protein, His-tagged | +Inquiry |
| ◆ Native Proteins | ||
| Luciferase-10V | Native Vibrio fischeri Luciferase | +Inquiry |
| Luciferase-09F | Active Native Firefly Luciferase | +Inquiry |
Case 1: Rahnama S, et al. Enzyme Microb Technol. 2017
In this research, researchers introduce an enhanced Renilla luciferase, super RLuc8, engineered for improved light emission and thermal stability. The study demonstrates that super RLuc8 exhibits a red-shifted emission spectrum and maintains consistent luminescence. Compared to the wild-type enzyme, super RLuc8 displays a significant 10-fold enhancement in thermostability at 37°C after a 20-minute incubation (p-value=0.0084). Its optimal operational temperature has also risen from 30 to 37°C. Molecular dynamics simulations suggest that this enhanced stability is likely due to improved structural compactness and increased local rigidity, particularly in areas away from the light-emitting center.
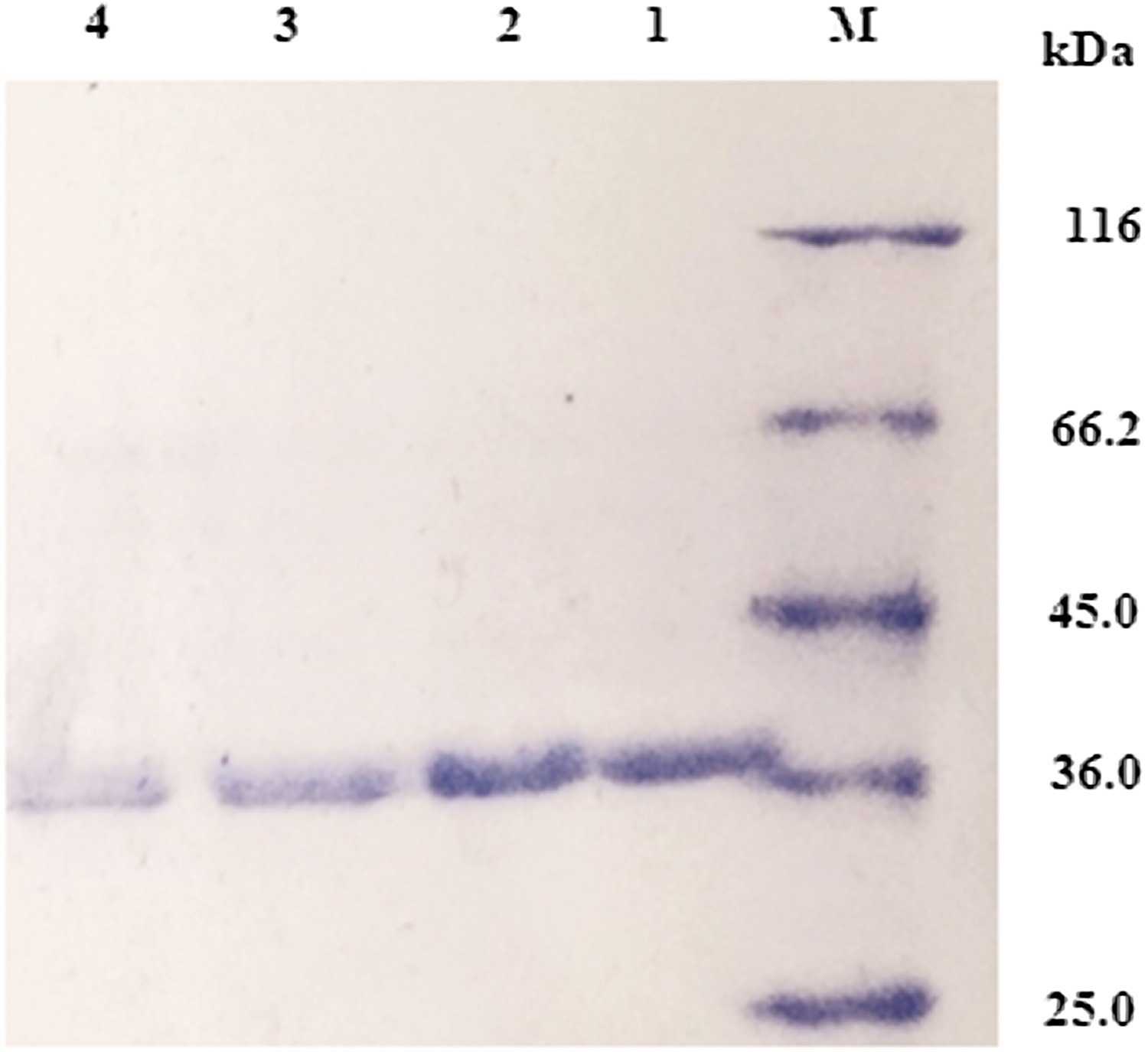
Fig1. SDS-PAGE analysis of purified protein fractions.
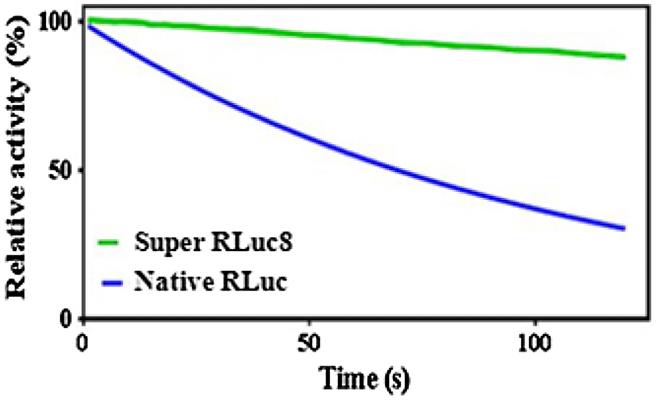
Fig2. Decay of light emission of native RLuc and super RLuc8.
Case 2: Inouye S, et al. Protein Expr Purif. 2013
Researchers utilized the cold-induced expression system in _Escherichia coli_ for the production of coelenterazine-dependent luciferases, including Renilla luciferase (RLase), its red-shifted variant (RLase-547), the catalytic domain of Oplophorus luciferase (19kOLase), and Gaussia luciferase (GLase). The luminescence characteristics of these purified luciferases were assessed using ten different C2-modified coelenterazine analogues. Under our experimental conditions, the native coelenterazine yielded the following maximum luminescence intensities: GLase (100%), RLase (8.0%), RLase-547 (0.73%), and 19kOLase (0.09%). Although the substrate specificities for the C2-modified analogues varied among the luciferases, the emission peaks remained unchanged with the C2-substituted coelenterazine. These findings imply that the enzymatic environment for coelenterazine oxidation and the excited state of coelenteramide may differ among these luciferases.
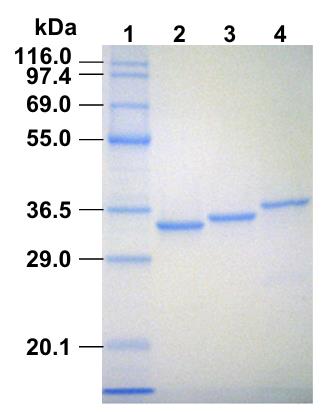
Fig1. SDS–PAGE analysis of purified Renilla luciferases from E. coli cells.
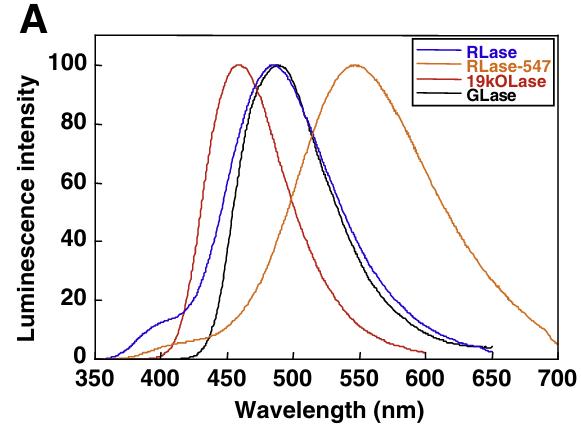
Fig2. Bioluminescence spectra of coelenterazine-type luciferases by reacting with coelenterazine.
Recombinant Renilla Luciferase Protein is a bioluminescent protein derived from the sea pansy, Renilla reniformis. It is extensively used as a reporter enzyme in various biological assays due to its stability, high signal-to-noise ratio, and lack of background activity in most biological systems. The protein catalyzes the oxidation of its substrate, coelenterazine, to produce light, which can be measured and used to infer the activity or expression of other genes in a system.
In the context of research and drug discovery, Renilla Luciferase is often used in dual-reporter assays alongside firefly luciferase. This allows for the normalization of transfection efficiency and other experimental variables, thus providing more accurate and reliable data. The development of red-shifted variants of Renilla luciferase has also enabled deeper tissue penetration for imaging applications in living subjects, expanding its utility in biological research and medicine.
Moreover, the protein has been engineered for enhanced stability and light output, making it more suitable for use in small animal imaging and other bioluminescent applications. These engineered versions offer improved resistance to inactivation in serum and increased luminescence, which can be beneficial for long-term studies and high-throughput screening.
In addition to its use as a research tool, Renilla Luciferase has been explored for its potential in gene therapy and as a biosensor for detecting specific biological molecules or environmental contaminants. Its applications span across various fields, including molecular and cellular biology, drug discovery, and even in the development of novel diagnostic tools.
The versatility of Renilla Luciferase, coupled with the ability to manipulate its properties through genetic engineering, positions it as a valuable asset in the life sciences toolkit. Its use in monitoring protein-protein interactions and as a sensitive reporter of gene expression makes it an important molecule in contemporary biological research.
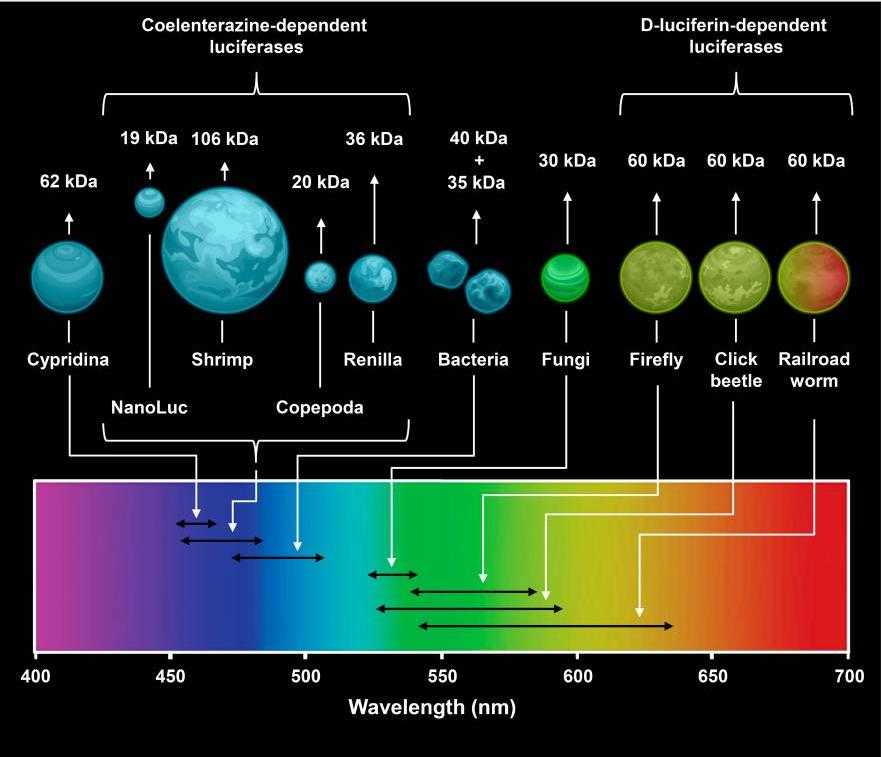
Fig1. Key properties of natural luciferases. (A. A. Kotlobay, 2020)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All Luciferase Products
Required fields are marked with *
My Review for All Luciferase Products
Required fields are marked with *
Inquiry Basket


