Recombinant Pyrococcus furiosus hydD Protein (M1-Q261)
| Cat.No. : | hydD-1386P |
| Product Overview : | Recombinant Pyrococcus furiosus hydD(1-261end) protein without tag was expressed in E. coli |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Pyrococcus furiosus |
| Source : | E.coli |
| Tag : | Non |
| ProteinLength : | M1-Q261 |
| Description : | Part of a bifunctional enzyme complex that functions as an NADPH-dependent hydrogen-evolving hydrogenase with sulfur reducing activity. May play a role in hydrogen cycling during fermentative growth. Activity not exhibited with NAD. The alpha and delta subunits form the hydrogenase component that catalyzes the reduction of protons to evolve hydrogen. |
| Form : | Liquid |
| Endotoxin : | < 0.01 EU/μg of the protein |
| Purity : | 90% |
| Stability : | Samples are stable for up to twelve months from date of receipt at -20 to -80 centigrade |
| Storage : | Store it under sterile conditions at -20 to -80 centigrade. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Storage Buffer : | Supplied as sterile 50 mM Tris-HCl (pH7.5), 200 mM NaCl, 20% glycerol |
| Shipping : | It is shipped out with blue ice. |
| Gene Name | PFDSM3638_RS04495 NADPH-dependent hydrogenase/sulfhydrogenase 1 subunit delta [ Pyrococcus furiosus DSM 3638 ] |
| Official Symbol | hydD |
| Synonyms | PFDSM3638_RS04495; NADPH-dependent hydrogenase/sulfhydrogenase 1 subunit delta; NADPH-dependent hydrogenase/sulfhydrogenase 1 subunit delta; EC 1.12.1.3 |
| Gene ID | 41712701 |
| Protein Refseq | WP_011012028 |
| UniProt ID | E7FHU4 |
| ◆ Recombinant Proteins | ||
| CFB-26762TH | Recombinant Human CFB, His-tagged | +Inquiry |
| IRAK4-54H | Recombinant Human IRAK4 protein, Flag-tagged, Biotinylated | +Inquiry |
| ANXA5-26600TH | Recombinant Human ANXA5 protein, His-tagged | +Inquiry |
| CLEC9A-02H | Active Recombinant Human CLEC9A protein, hFc-tagged | +Inquiry |
| RFL24489GF | Recombinant Full Length Chicken Frizzled-4(Fzd4) Protein, His-Tagged | +Inquiry |
| ◆ Native Proteins | ||
| CEACAM5-27803TH | Native Human CEACAM5 | +Inquiry |
| HSV1Ag-354H | Active Native Herpes Simplex Virus 1 Protein | +Inquiry |
| MFGE8-8518B | Native Bovine MFGE8, Fluoresence-labeled | +Inquiry |
| Lectin-1744M | Active Native Maclura Pomifera Lectin Protein | +Inquiry |
| Lectin-1780G | Active Native Griffonia Simplicifolia Lectin I Protein, Fluorescein labeled | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| UHRF1-508HCL | Recombinant Human UHRF1 293 Cell Lysate | +Inquiry |
| TMEM138-1002HCL | Recombinant Human TMEM138 293 Cell Lysate | +Inquiry |
| OSBPL11-3541HCL | Recombinant Human OSBPL11 293 Cell Lysate | +Inquiry |
| SKI-1815HCL | Recombinant Human SKI 293 Cell Lysate | +Inquiry |
| SF1-1922HCL | Recombinant Human SF1 293 Cell Lysate | +Inquiry |
Case 1: Rieckenberg F, et al. Bioprocess Biosyst Eng. 2014
Pyrococcus furiosus, a thermophilic archaeon, is valuable for its NADPH-dependent hydrogenase I but challenging to cultivate at scale due to oxygen sensitivity and rapid lysis. Researchers improved P. furiosus biomass production and hydrogenase I purification by optimizing the fermentation process in a 300-L bioreactor. Key enhancements included an unsterile repeated-batch cultivation method, streamlined inoculation, precise harvesting timing, and a simplified one-step enzyme purification, resulting in a robust and efficient production system.
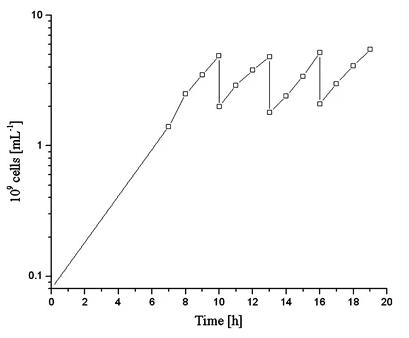
Fig1. Repeated-batch fermentations (medium B) including a high frequent harvesting.

Fig2. Results of one-step purification of hydrogenase I using 9 g of P. furiosus wet biomass.
Case 2: Chandrayan SK, et al. J Biol Chem. 2012
The hyperthermophilic archaeon Pyrococcus furiosus produces cytoplasmic hydrogenase (SHI), a promising enzyme for bioenergy applications. However, scaling up its production has been challenging. Researchers engineered P. furiosus for SHI overproduction using a strong promoter and a Strep-tag II for purification. The modified strain showed 20-fold higher SHI mRNA levels and approximately 10-fold greater specific activity compared to the wild type while maintaining normal growth and hydrogen production rates. The overexpressed SHI was efficiently purified and exhibited properties similar to the native enzyme. This advancement makes P. furiosus a superior platform for SHI production, potentially boosting in vitro biohydrogen generation.
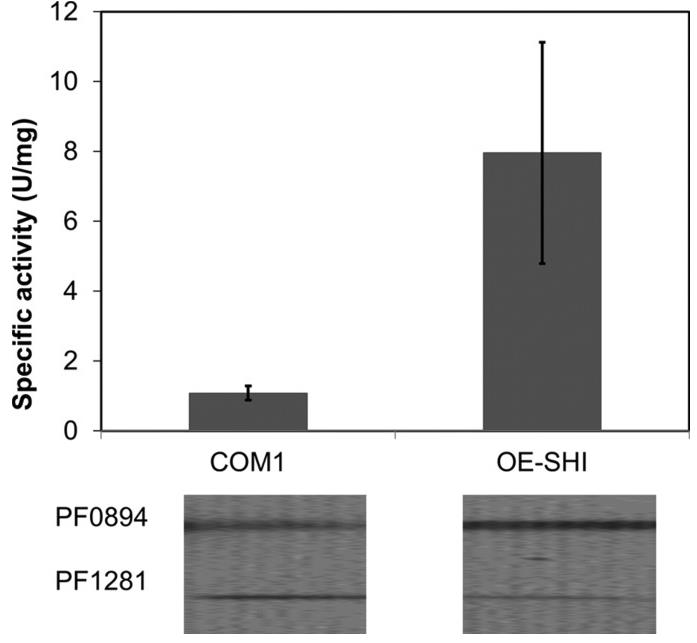
Fig1. Increased catalytic activity and amount of catalytic subunit of SHI in OE-SHI strain.
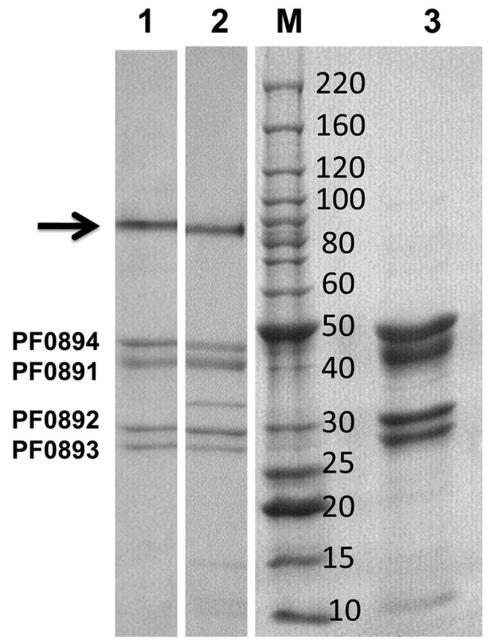
Fig2. Electrophoretic analysis of OE-SHI hydrogenase.
Recombinant Pyrococcus furiosus hydD Protein is a recombinant protein derived from extremely thermophilic bacterium Pyrococcus furiosus, which has special significance and application value in biological research.
It has several biological functions, including:
Thermal stability: As a protein from extremely thermophilic organisms, hydD protein has high thermal stability and can maintain its structure and function in high temperature environments.
Molecular chaperone: It may be involved in the correct folding of proteins and preventing polymerization, especially under high temperature conditions.
Based on biological function, the protein currently has a variety of practical applications, such as:
Improve the thermal stability of other proteins: By fusing with target proteins, hydD proteins can improve the thermal stability of these proteins, increasing their potential for industrial applications.
Biotechnology and pharmaceuticals: In biotechnology and pharmaceutical processes that require high-temperature treatment, the use of this protein can help improve the stability and efficiency of enzymes and other biocatalysts.
Basic research: The hydD protein provides an important research tool in studying how proteins remain active under extreme conditions.
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All hydD Products
Required fields are marked with *
My Review for All hydD Products
Required fields are marked with *
Inquiry Basket


