Recombinant Human TMPRSS2 Protein (106-492 aa), His-tagged
| Cat.No. : | TMPRSS2-1856H |
| Product Overview : | Recombinant Human TMPRSS2 Protein (106-492 aa) is produced by Yeast expression system. This protein is fused with a 6xHis tag at the N-terminal. Protein Description: Partial. |
| Availability | April 19, 2025 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | Yeast |
| Tag : | His |
| Protein Length : | 106-492 aa |
| Description : | Serine protease that proteolytically cleaves and activates the viral spike glycoproteins which facilitate virus-cell membrane fusions; spike proteins are synthesized and maintained in precursor intermediate folding states and proteolysis permits the refolding and energy release required to create stable virus-cell linkages and membrane coalescence. Facilitates human SARS coronavirus (SARS-CoV) infection via two independent mechanisms, proteolytic cleavage of ACE2, which might promote viral uptake, and cleavage of coronavirus spike glycoprotein which activates the glycoprotein for cathepsin L-independent host cell entry. Proteolytically cleaves and activates the spike glycoproteins of human coronavirus 229E (HCoV-229E) and human coronavirus EMC (HCoV-EMC) and the fusion glycoproteins F0 of Sendai virus (SeV), human metapneumovirus (HMPV), human parainfluenza 1, 2, 3, 4a and 4b viruses (HPIV). Essential for spread and pathogenesis of influenza A virus (strains H1N1, H3N2 and H7N9); involved in proteolytic cleavage and activation of hemagglutinin (HA) protein which is essential for viral infectivity. |
| Form : | Tris-based buffer,50% glycerol |
| Molecular Mass : | 44.8 kDa |
| AA Sequence : | WKFMGSKCSNSGIECDSSGTCINPSNWCDGVSHCPGGEDENRCVRLYGPNFILQVYSSQRKSWHPVCQDDWNENYGRAACRDMGYKNNFYSSQGIVDDSGSTSFMKLNTSAGNVDIYKKLYHSDACSSKAVVSLRCIACGVNLNSSRQSRIVGGESALPGAWPWQVSLHVQNVHVCGGSIITPEWIVTAAHCVEKPLNNPWHWTAFAGILRQSFMFYGAGYQVEKVISHPNYDSKTKNNDIALMKLQKPLTFNDLVKPVCLPNPGMMLQPEQLCWISGWGATEEKGKTSEVLNAAKVLLIETQRCNSRYVYDNLITPAMICAGFLQGNVDSCQGDSGGPLVTSKNNIWWLIGDTSWGSGCAKAYRPGVYGNVMVFTDWIYRQMRADG |
| Purity : | > 90% as determined by SDS-PAGE. |
| Notes : | Repeated freezing and thawing is not recommended. Store working aliquots at 4 centigrade for up to one week. |
| Storage : | The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20 centigrade/-80 centigrade. The shelf life of lyophilized form is 12 months at -20 centigrade/-80 centigrade. |
| Concentration : | A hardcopy of COA with reconstitution instruction is sent along with the products. |
| Publications : |
An Enzymatic TMPRSS2 Assay for Assessment of Clinical Candidates and Discovery of Inhibitors as Potential Treatment of COVID-19 (2020)
Electronic Supplementary Information for The ACE2 receptor accelerates but is not biochemically required for SARS-CoV-2 membrane fusion (2023)
Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions (2021)
Discovery and characterization of protease inhibitors that block SARS-CoV-2 infection (2023)
Factor Xa cleaves SARS-CoV-2 spike protein to block viral entry and infection (2023)
Peptidomimetic inhibitors of TMPRSS2 block SARS-CoV-2 infection in cell culture (2022)
Molecular Interactions of Tannic Acid with Proteins Associated with SARS-CoV-2 Infectivity (2021)
Improving the selectivity of 3-amidinophenylalanine-derived matriptase inhibitors (2022)
Alpha-1 antitrypsin inhibits TMPRSS2 protease activity and SARS-CoV-2 infection (2021)
Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry (2021)
Hesperidin Is a Potential Inhibitor against SARS-CoV-2 Infection (2021)
Simultaneous Inhibition of SARS-CoV-2 Infectivity by a Specific Combination of Plant-derived Compounds (2021)
FXa cleaves the SARS-CoV-2 spike protein and blocks cell entry to protect against infection with inferior effects in B.1.1.7 variant (2021)
Suite of TMPRSS2 Assays for Screening Drug Repurposing Candidates as Potential Treatments of COVID-19 (2022)
Quercetin and taxifolin inhibits TMPRSS2 activity and its interaction with EGFR in paclitaxel-resistant breast cancer cells: An in silico and in vitro study. (2024)
High-throughput amino acid-level characterization of the interactions of plasminogen activator inhibitor-1 with variably divergent proteases (2024)
|
| Gene Name | TMPRSS2 transmembrane protease, serine 2 [ Homo sapiens ] |
| Official Symbol | TMPRSS2 |
| Synonyms | TMPRSS2; PRSS10; epitheliasin; serine protease 10; PP9284; FLJ41954; |
| Gene ID | 7113 |
| mRNA Refseq | NM_001135099 |
| Protein Refseq | NP_001128571 |
| MIM | 602060 |
| UniProt ID | O15393 |
| ◆ Recombinant Proteins | ||
| Tmprss2-429R | Recombinant Rat Tmprss2 Protein, His-tagged | +Inquiry |
| TMPRSS2-6852HF | Recombinant Full Length Human TMPRSS2 Protein, GST-tagged | +Inquiry |
| TMPRSS2-4899H | Recombinant Human TMPRSS2 protein, His-tagged | +Inquiry |
| TMPRSS2-6469H | Recombinant Human TMPRSS2 Protein (Ser284-Gly492), His tagged | +Inquiry |
| TMPRSS2-4598H | Recombinant Human TMPRSS2 protein, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| TMPRSS2-910HCL | Recombinant Human TMPRSS2 293 Cell Lysate | +Inquiry |
Case 1: Wettstein L, et al. Nat Commun. 2021
SARS-CoV-2 mainly infects respiratory epithelial cells. To better understand the innate immunity of the respiratory tract to SARS-CoV-2, researchers generated and screened a peptide/protein library from bronchoalveolar lavage fluid for inhibitors that inhibit SARS-CoV-2 spike-driven entry. They found that α1-antitrypsin (α1AT), an abundant circulating serine protease inhibitor, inhibited the entry of SARS-CoV-2 at physiological concentrations and inhibited viral replication in cell lines and primary cells, including human respiratory epithelial cultures. Then researchers further demonstrated that α1AT binds to and inactivates the serine protease TMPRSS2, which is an essential enzyme for membrane fusion of SARS-CoV-2 spinous protein. Therefore, α1AT, as an acute phase protein, not only inhibits TMPRSS2, but also inhibits the entry of SARS-CoV-2, which may play an important role in innate immune defense against novel coronavirus.
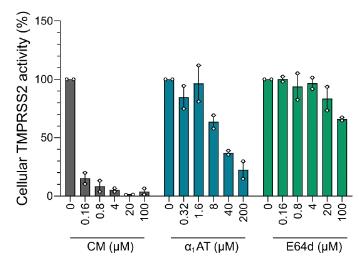
Fig1. α1AT inhibits cell-associated TMPRSS2 activity.
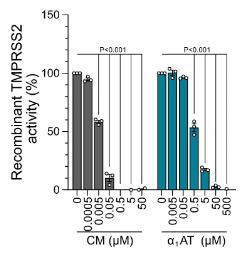
Fig2. α1AT inhibits recombinant TMPRSS2 enzyme activity.
Case 2: Shrimp JH, et al. ACS Infect Dis. 2022
An effective early intervention against SARS-CoV-2 involves blocking viral entry, which occurs when the virus's spike protein binds to ACE2 receptors on host cells and is then cleaved by TMPRSS2. Inhibiting TMPRSS2's protease activity could offer a promising therapeutic approach.
Researchers conducted a high-throughput screening of 6030 compounds using a fluorogenic assay, followed by an orthogonal assay with mass spectrometry to validate hits. The most promising candidates were then tested in a cell-based assay using SARS-CoV-2 pseudotyped particles.
From this process, six molecules were selected for further research, including two approved Japanese drugs (camostat and nafamostat), two in clinical trials (PCI-27483 and otamixaban), and two peptidomimetic inhibitors of TMPRSS2 from literature that haven't entered trials (compounds 92 and 114). This research outlines a comprehensive strategy for identifying and developing new TMPRSS2 inhibitors as potential antivirals.
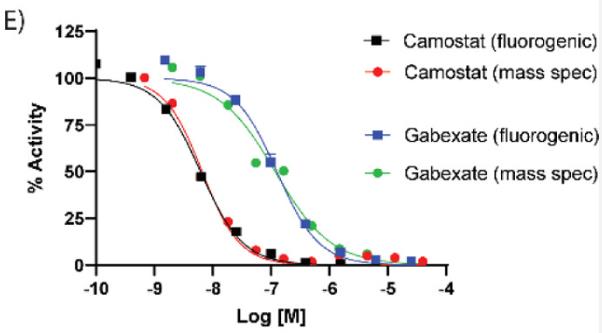
Fig1. Comparison of TMPRSS2 fluorogenic detection and mass spectrometry detection assays by assessing dose–response inhibition by camostat and gabexate.
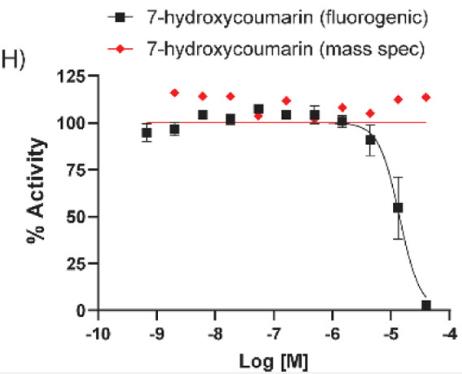
Fig2. Dose–response inhibition of 7-hydroxycoumarin against TMPRSS2.
Case 3: Wettstein L, et al. Commun Biol. 2022
TMPRSS2 is a key enzyme for SARS-CoV-2 Spike protein activation, making it a potential therapeutic target for COVID-19. Researchers developed peptidomimetic inhibitors of TMPRSS2 using in silico molecular docking to identify catalytic site binders. These compounds were synthesized with a serine trap, showing selectivity for TMPRSS2 over other proteases and stability in blood serum and plasma for over ten days.
The lead peptidomimetic inhibitors effectively prevented SARS-CoV-2 pseudovirus entry and authentic virus infection, matching the efficacy of camostat mesylate. Importantly, they also blocked entry of concerning variants Delta and Omicron BA.1. This work presents the successful design and characterization of peptidomimetic TMPRSS2 inhibitors as potential broad-spectrum antivirals against SARS-CoV-2 and its variants.
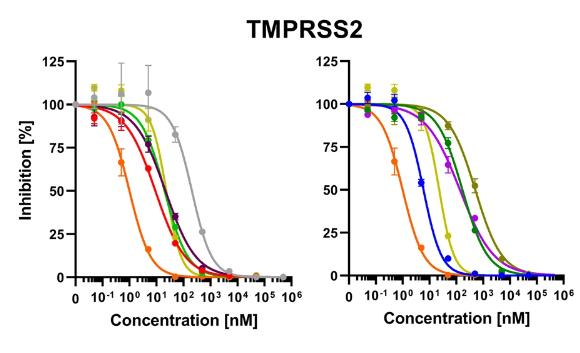
Fig1. Isolated TMPRSS2 was mixed with peptidomimetic inhibitors, camostat mesylate (CM), and FOY-251.
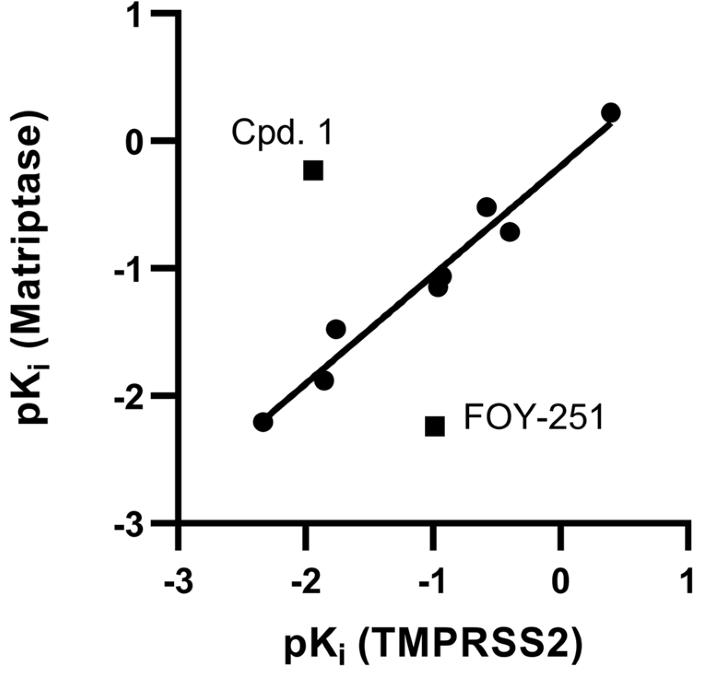
Fig2. Correlation of the pKi values from compounds 1–8, CM and FOY-251 against TMPRSS2 and matriptase.
TMPRSS2, or transmembrane serine protease 2, is a protein that plays an important role in a variety of physiological and pathological processes. In particular, during SARS-CoV-2 virus infection, TMPRSS2 promotes the processing of viral Spike (S) protein through its enzyme activity, which is the key step for the virus to enter the host cell and cause infection.
Due to its role in SARS-CoV-2 infection, TMPRSS2 has become a target for the development of antiviral therapeutics against COVID-19. Studies have explored the potential of using clinically known serine protease inhibitors such as nafamostat and camostat as COVID-19 therapeutics, which block viral activity by targeting TMPRSS2. Through engineering and crystallization, the X-ray crystal structure of TMPRSS2 can be determined, which helps to understand its active site and substrate binding properties, and provides a structural basis for the design of specific inhibitors.
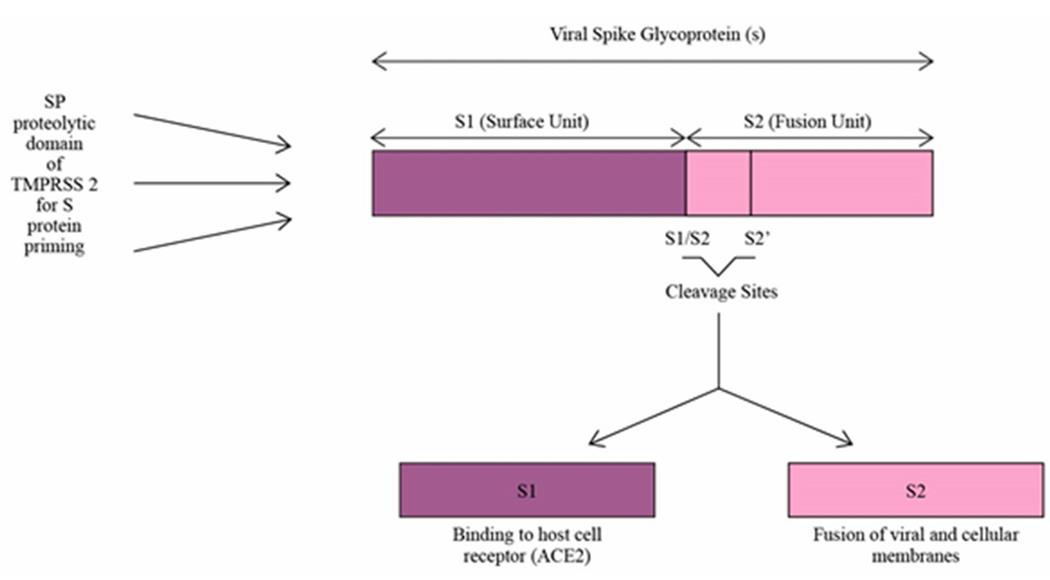
Fig1. S protein priming of SARS-CoV-2 by SP proteolytic domain of TMPRSS2. (Datesh Daneshwar, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All TMPRSS2 Products
Required fields are marked with *
My Review for All TMPRSS2 Products
Required fields are marked with *
Inquiry Basket


