Recombinant Human RPA complex
| Cat.No. : | RPA-1536H |
| Product Overview : | Recombinant human RPA complex was expressed in and purified from E. coli. |
| Availability | March 15, 2025 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | Non |
| Description : | RPA is a protein complex consisting of 3 subunits, RPA1, RPA2, and RPA3. RPA is the single-stranded DNA binding protein in eukaryotes. It functions in multiple cellular processes including replication, recombination, and DNA repair. |
| Form : | 30 mM HEPES (pH7.8), 1 mM DTT, 0.25 mM EDTA, 300 mM KCl, 0.25% (W/V) myo-inositol, and 0.01%(V/V) Nonidet P-40 |
| Molecular Mass : | RPA1 (70 kDa); RPA2 (32 kDa); RPA3 (14 kDa) |
| Storage : | Stable for 2 years at -70 centigrade from date of shipment. Please aliquot to avoid repeated freezing and thawing. |
| Gene Name | RPA1 replication protein A1 [ Homo sapiens (human) ] |
| Official Symbol | RPA |
| Synonyms | HSSB; RF-A; RP-A; REPA1; RPA70; MST075 |
| Gene ID | 6117 |
| mRNA Refseq | NM_002945.3 |
| Protein Refseq | NP_002936.1 |
| MIM | 179835 |
| UniProt ID | P27694 |
| ◆ Recombinant Proteins | ||
| RPA-1536H | Recombinant Human RPA complex | +Inquiry |
| RPA-264S | Recombinant Staphylococcus RPA, His-tagged, Biotinylated | +Inquiry |
Case 1: Mazina OM, et al. J Biol Chem. 2020
Replication protein A (RPA), crucial for DNA processes involving single-stranded DNA (ssDNA) such as replication, repair, and signaling, is known for its strong ssDNA binding. Contrary to previous beliefs of its weak RNA binding, recent findings indicate RPA's potential involvement in RNA metabolism and R-loop formation, which are prevalent in both prokaryotic and eukaryotic genomes and significant for recombination and replication restart. The mechanism behind R-loop formation is still unclear.
This study explored human RPA's RNA-binding capacity and its role in R-loop formation. Through gel-retardation and competition assays, researchers discovered RPA's surprisingly high affinity for RNA (KD ≈ 100 pm) and its ability to facilitate R-loop formation with double-stranded DNA. Experiments demonstrated that human DNA polymerases can initiate DNA synthesis using RPA-generated R-loops, simulating the replication restart process in vivo.
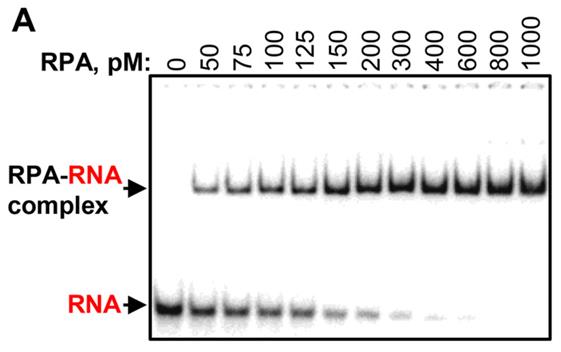
Fig1. Analysis of RPA binding to a 48-mer RNA.
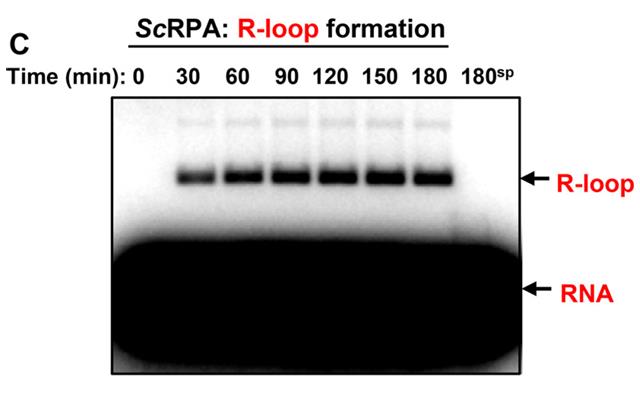
Fig2. Kinetics of R-loop formation by ScRPA (100 nm) between 48-mer RNA and pUC19 dsDNA analyzed by electrophoresis in a 1% agarose gel.
Case 2: Qin Z, et al. Elife. 2020
The BLM helicase is vital for genome stability, handling various DNA substrates in replication and repair, except for nicked DNA. The mechanism by which BLM adapts to its multiple roles is not well understood. This single-molecule study reveals that a high concentration of BLM can unwind double-stranded DNA from a nick under tension. Remarkably, human replication protein A (hRPA) enhances BLM's ability to unwind nicked DNA under lower force and enables BLM to move on both intact and nicked single-stranded DNAs, achieving bidirectional unwinding. This activation relies on BLM's targeting to the nick and the availability of free hRPAs, without the need for direct interaction between them.
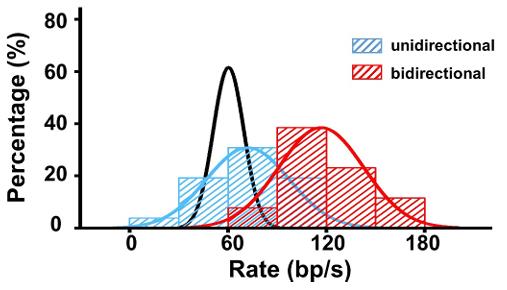
Fig1. Distributions of the uni- and bi- directional dsDNA unwinding rates in the presence of 200 nM BLM and 50 nM RPA under 30 pN.
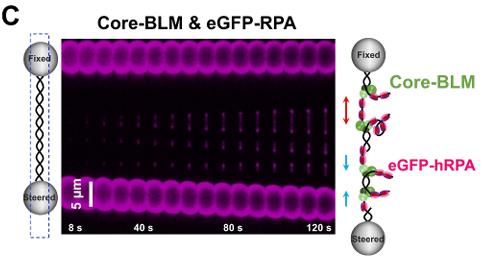
Fig2. A representative kymograph of a single DNA tether unwound in the presence of 20 nM core-BLM and 50 nM eGFP-RPA.
The Human RPA complex, or Human replicating protein A complex, is a protein complex that plays a key role in DNA replication and repair. It consists of three subunits: RPA1, RPA2, and RPA3, which together form a stable heterotrimer structure. The main function of the RPA complex during DNA replication is to protect single-stranded DNA (ssDNA) and prevent it from forming secondary structures, while also contributing to the stability of double-stranded DNA. In addition, RPA is involved in regulating the initiation and termination of DNA replication, as well as interacting with a variety of proteins, such as DNA polymerase, DNA helicase and DNA ligase, to ensure the smooth progress of DNA replication.
In the field of cancer treatment, the research of RPA complexes has also made important progress. Because of its central role in DNA metabolism, RPA is an attractive option for cancer therapy targets. For example, by regulating post-translational modifications of RPA, such as phosphorylation and acetylation, it can affect its function in the DNA damage response, thereby inhibiting the growth of tumor cells. These findings provide new ideas for the development of new anticancer drugs, especially those that target specific RPA-modified states.
In addition, in the research and treatment of genetic diseases, the application of RPA complexes also shows great potential. Through high-resolution genome mapping, researchers have identified the locations of three RPA genes (RPA1, RPA2, and RPA3), which helps elucidate the biochemical basis of genetic diseases related to DNA metabolism. For example, RPA recombinant products synthesized in vitro can simulate different DNA damage and repair processes, helping scientists to better understand the mechanisms by which these diseases occur and develop corresponding treatments.
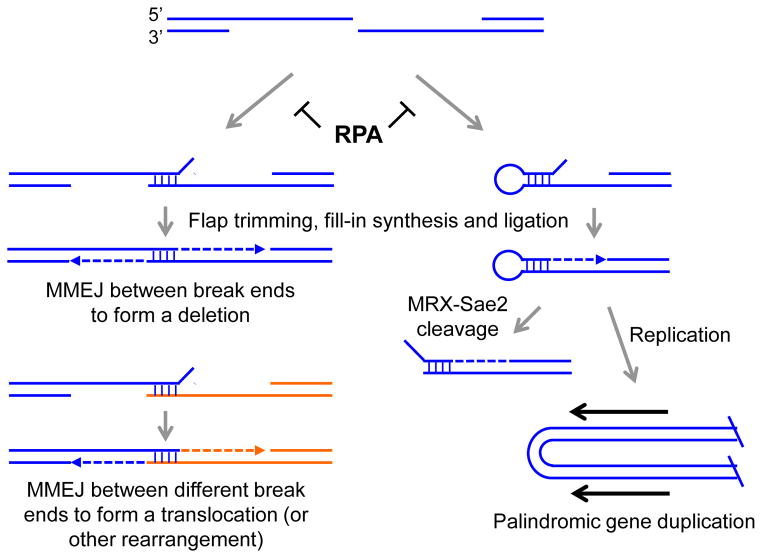
Fig1. RPA prevents inter- and intra-molecular annealing between short repeats to prevent MMEJ or formation of hairpin-capped DNA ends. (Sarah K Deng, 2015)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All RPA Products
Required fields are marked with *
My Review for All RPA Products
Required fields are marked with *
Inquiry Basket


