Recombinant Human EBAG9 protein(Arg28-Ser213), His-tagged
| Cat.No. : | EBAG9-457H |
| Product Overview : | Recombinant Human EBAG9 (O00559) (Arg28-Ser213) was expressed in E. coli with a polyhistidine tag at the N-terminus. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | 28-213 a.a. |
| Form : | Lyophilized from sterile PBS, pH 7.4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human EBAG9 consists of 201 amino acids and predicts a molecular mass of 23 KDa. It migrates as an approximately 33 KDa band in SDS-PAGE under reducing conditions. |
| Purity : | > 90 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | EBAG9 estrogen receptor binding site associated, antigen, 9 [ Homo sapiens ] |
| Official Symbol | EBAG9 |
| Synonyms | EBAG9; estrogen receptor binding site associated, antigen, 9; receptor-binding cancer antigen expressed on SiSo cells; EB9; RCAS1; cancer associated surface antigen; cancer-associated surface antigen RCAS1; estrogen receptor-binding fragment-associated gene 9 protein; PDAF; |
| Gene ID | 9166 |
| mRNA Refseq | NM_004215 |
| Protein Refseq | NP_004206 |
| MIM | 605772 |
| UniProt ID | O00559 |
| ◆ Recombinant Proteins | ||
| EBAG9-385H | Recombinant Human Estrogen Receptor Binding Site Associated, Antigen, 9, His-tagged | +Inquiry |
| EBAG9-5867H | Recombinant Human EBAG9 Protein, Myc/DDK-tagged, C13 and N15-labeled | +Inquiry |
| EBAG9-3017H | Recombinant Human EBAG9 Protein, GST-tagged | +Inquiry |
| EBAG9-1997R | Recombinant Rat EBAG9 Protein | +Inquiry |
| EBAG9-26510TH | Recombinant Human EBAG9, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| EBAG9-6737HCL | Recombinant Human EBAG9 293 Cell Lysate | +Inquiry |
Case 1: Wirges A, et al. Mol Ther. 2022
EBAG9 silencing enhances CAR T-cell efficacy by counteracting its immune checkpoint role in cytolytic enzyme suppression. miRNA-driven EBAG9 knockdown boosts tumor eradication in xenotransplant models, requiring lower therapeutic doses without elevating cytokine release syndrome risks. Integrated retroviral CAR/TCR-EBAG9 silencing streamlines manufacturing, supporting safer, high-efficacy adoptive T-cell therapies.
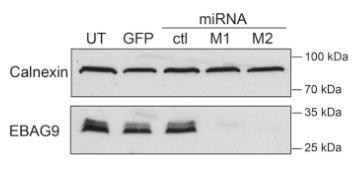
Fig1. RNAi-mediated reduction of EBAG9 protein level.
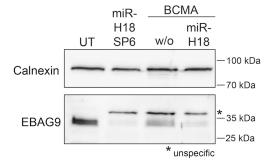
Fig2. Western blot of EBAG9 protein expression in sorted CD8+ IgG+ cells.
Case 2: Tanaka H, et al. J Transl Med. 2014
RCAS1 promotes immune evasion in oral squamous cell carcinoma (OSCC) by inducing lymphocyte apoptosis via membrane-bound and soluble forms. Overexpressed in metastatic SQUU-B cells, RCAS1 enhances apoptosis in co-cultured K562 leukemia cells, while knockdown reduces this effect. Soluble RCAS1 retains pro-apoptotic activity, highlighting its role in tumor microenvironment immune suppression.
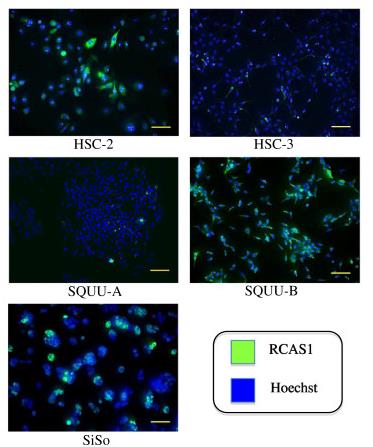
Fig1. RCAS1 protein was diffusely expressed in both cytoplasm and on membranes in all OSCC cell lines and SiSo, and was especially strong in SQUU-B cells.
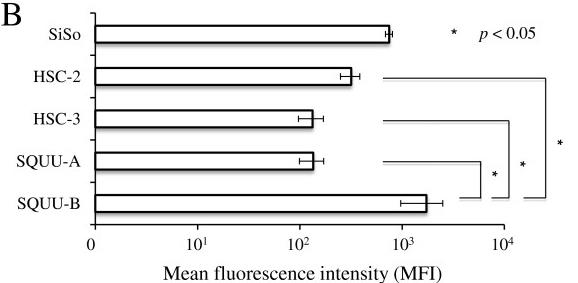
Fig2. Mean fluorescence intensity (MFI) of membranous RCAS1 was significantly higher in SQUU-B than in the other 3 OSCC cell lines.
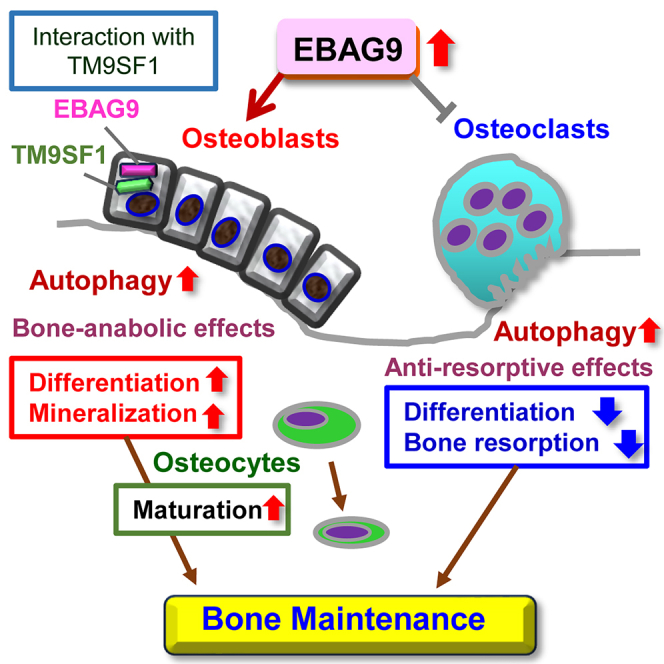
Fig1. EBAG9 plays a physiological role in bone maintenance by promoting autophagy together with its interactor TM9SF1. (Kotaro Azuma, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All EBAG9 Products
Required fields are marked with *
My Review for All EBAG9 Products
Required fields are marked with *
Inquiry Basket


