Recombinant Human ATP6V1F protein(Met1-Arg119), GST-tagged
| Cat.No. : | ATP6V1F-10048H |
| Product Overview : | Recombinant Human ATP6V1F (NP_004222.2) (Met1-Arg119) was expressed in E. coli with the GST tag at the N-terminus. |
| Availability | April 19, 2025 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | GST |
| Protein Length : | Met1-Arg119 |
| Form : | Lyophilized from sterile PBS, pH 7.4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human ATP6V1F/GST chimera consists of 350 amino acids and has a predicted molecular mass of 40.2 KDa. It migrates as an approximately 40 KDa band in SDS-PAGE under reducing conditions. |
| Purity : | > 90 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | ATP6V1F ATPase, H+ transporting, lysosomal 14kDa, V1 subunit F [ Homo sapiens ] |
| Official Symbol | ATP6V1F |
| Synonyms | ATP6V1F; ATPase, H+ transporting, lysosomal 14kDa, V1 subunit F; V-type proton ATPase subunit F; ATP6S14; VATF; Vma7; V-ATPase F subunit; V-ATPase subunit F; ATPase, vacuolar, 14 kD; V-ATPase 14 kDa subunit; vacuolar proton pump F subunit; vacuolar proton pump subunit F; vacuolar ATP synthase subunit F; adenosinetriphosphatase 14k chain; H(+)-transporting two-sector ATPase, 14kD subunit; MGC117321; MGC126037; MGC126038; |
| Gene ID | 9296 |
| mRNA Refseq | NM_001198909 |
| Protein Refseq | NP_001185838 |
| MIM | 607160 |
| UniProt ID | Q16864 |
| ◆ Recombinant Proteins | ||
| Atp6v1f-1774M | Recombinant Mouse Atp6v1f Protein, Myc/DDK-tagged | +Inquiry |
| ATP6V1F-550R | Recombinant Rat ATP6V1F Protein, His (Fc)-Avi-tagged | +Inquiry |
| ATP6V1F-1115HF | Recombinant Full Length Human ATP6V1F Protein, GST-tagged | +Inquiry |
| ATP6V1F-1008H | Recombinant Human ATP6V1F protein, GST-tagged | +Inquiry |
| ATP6V1F-894R | Recombinant Rat ATP6V1F Protein | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| ATP6V1F-8578HCL | Recombinant Human ATP6V1F 293 Cell Lysate | +Inquiry |
Case 1: Smith AN, et al. J Bioenerg Biomembr. 2008
The vacuolar H(+)-ATPase is made up of two parts: the V(1) domain, which breaks down ATP, and the V(0) domain, which moves hydrogen ions around. In mammals, there's not much known about the V(0) d subunit, especially what exactly it does and where it stays within the pump. It has two forms from different genes: d1 is found everywhere, while d2 mostly shows up on the cell surface in kidneys and osteoclasts. To find out if it's a core part of the central stalk like in bacterial pumps or sits on the outside as yeast research suggests, scientists looked at both human isoforms. Model simulations showed that even though human d1 and d2 don't share much sequence similarity to the bacterial subunit C, they structure similarly. Further studies revealed that both d1 and d2 can interact with and pull down the central stalk's D and F subunits from kidney membranes, proving that their connection is straightforward.
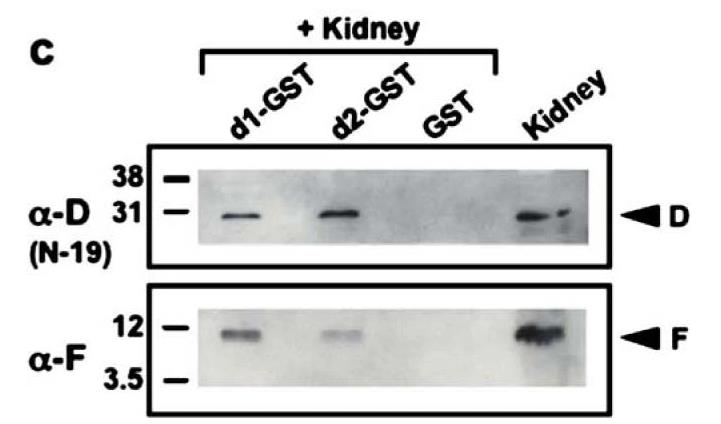
Fig1. Immunoblotting using specific anti-D (N-19) and anti-F antisera shows that d1-GST or d2-GST, but not GST alone are able to pull down the D and F subunits from human kidney membrane preparations.
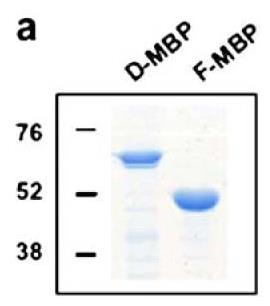
Fig2. SDS-PAGE analysis of affinity purified D-MBP and F-MBP shows that each runs as a single major species.
Case 2: Sharma S, et al. J Biol Chem. 2018
Vacuolar H±ATPases, or V-ATPases, are specialized pumps that help regulate the acidity inside cells and sometimes outside in certain tissues. They work like tiny motors, spinning to move protons and are controlled by a process where they fall apart into inactive pieces and then come back together. One of the key components of this pump is subunit H. It's important because it helps with energy production and keeps the pump stable when it's not attached to the membrane. Scientists looked into how this subunit interacts with other parts of the pump by measuring how strongly it binds to specific sections using advanced techniques. They found that subunit H can bind strongly to some parts but not all, revealing a kind of imbalance in how the pump parts come together. Interestingly, when the pump is working hard, the bond between subunit H and the pump weakens, which links back to how the pump turns energy into movement and controls its function.
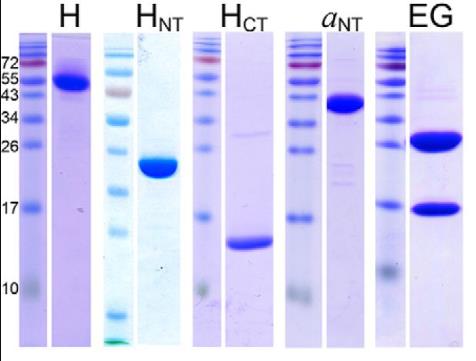
Fig1. SDS-polyacrylamide gel of purified recombinant proteins used in the interaction studies.
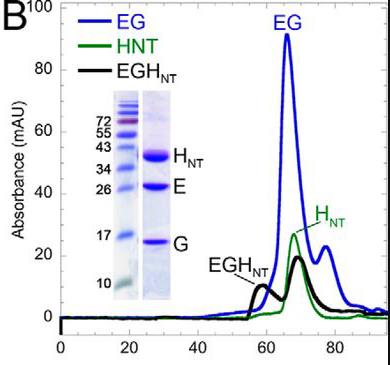
Fig2. The ITC cell content was subjected to gel filtration on a 1.6 × 50-cm Superdex 200 column.
Recombinant Human ATP6V1F (rhATP6V1F) is a key player in managing cell pH levels as part of the V-ATPase V1 complex. In research, it's used to explore how cells regulate pH and the functions of V-ATPase. This enzyme is essential for acidifying cell compartments, impacting things like endocytosis and neurotransmitter release. Scientists rely on rhATP6V1F to study how the V1 complex assembles and functions, and it's also used in various assays, such as ELISA and Western Blot, to understand protein interactions and expression levels.
On the industrial side, rhATP6V1F is produced for use in drug development and diagnostics, thanks to its consistent production in E. coli. This consistency makes it a reliable resource for developing treatments for diseases linked to pH imbalances, like some cancers. Its involvement in cell processes also makes rhATP6V1F a valuable target in drug discovery. The industrial production ensures a stable supply for creating standardized research reagents, helping maintain the accuracy of scientific research.
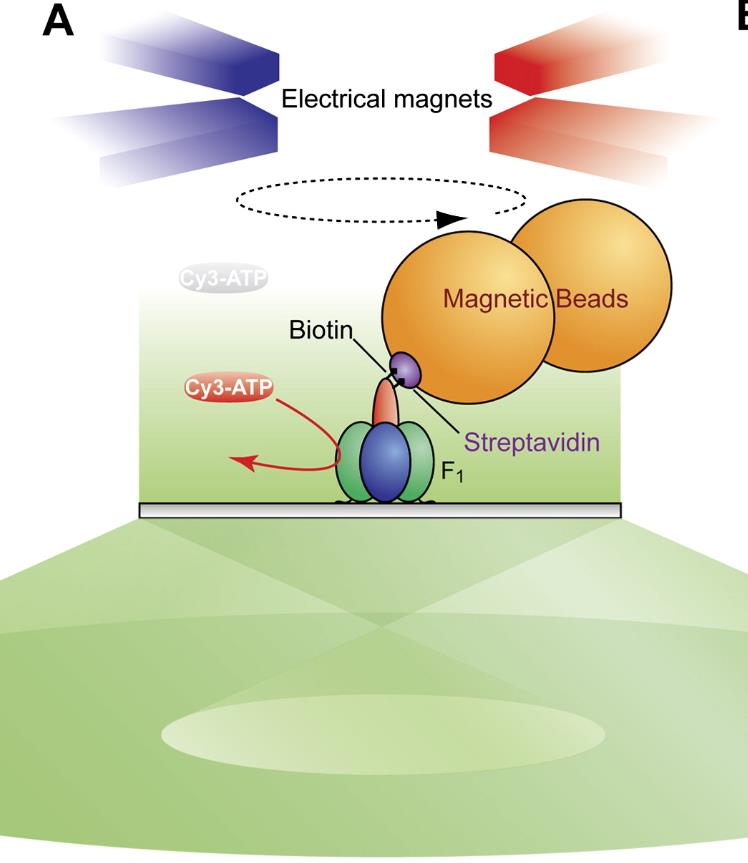
Fig1. Angle between Binding and Release of Cy3-ATP during Controlled Rotation. (Kengo Adachi, 2007)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All ATP6V1F Products
Required fields are marked with *
My Review for All ATP6V1F Products
Required fields are marked with *
Inquiry Basket


