Recombinant Haemophilus influenzae infB Protein
| Cat.No. : | infB-112H |
| Product Overview : | Recombinant Haemophilus influenzae infB Protein was expressed in E. coli. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Haemophilus influenzae |
| Source : | E.coli |
| Description : | Translation initiation factor IF-2 |
| Form : | Liquid. In 50 mM NaH2PO4 buffer (pH8.0) containing 300 mM NaCl. |
| Molecular Mass : | ~94.4 kDa |
| AA Sequence : | MTEDVKADAPKKLSIQRRTKTTVSSTTTGGKSKEVQVEVRKKRTVKTDIAQQEEAKLKAQQEAEAKKIAEQKAAEEKARLEAEKAATKKEADEKSKAEKAKAETAKPAKSAVDSKAKFVDPEKEKRKAEEAELRRKAEEVARQKAEEQARRAAEEAKRYAEADDSDNESSSEDYSDYNLSSRYALEAEDEEDRRNENRGRGKNKVAKAKKGGRDDENSKNSKNERESNRKNQKDAKFGKGKNGKKGTALQQAFTKPVQVVKADVVIGETITVAELANKMSVKATEIIKVMMKMGEMVTINQVIDQETAQLVAEELGHKVILRNENELEEAVLGDRDVNAEKVTRAPVVTIMGHVDHGKTSLLDYIRKAKVAAGEAGGITQHIGAYHVEMDDGKMITFLDTPGHAAFTSMRARGAKATDIVVLVVAADDGVMPQTIEAIQHAKAAGAPLVVAVNKIDKPEANPDRVEQELLQHDVISEKFGGDVQFVPVSAKKGTGVDDLLDAILLQSEVLELTAVKDGMASGVVIESYLDKGRGPVATILVQSGTLRKGDIVLCGFEYGRARAMRDENGKEVDEAGPSIPVELLGLSGVPAAGDEATVVRDEKKAREVALYRQGKFREVKLARQQKAKLENMFSNMSEGDVAELNVIVKADVQGSVEAIVQALNELSTNEVKVKVVGSGVGGITETDATLATASNAIIVGFNVRADATARRVIEAENIDLRYYSIIYELLNEIKAAMSGMLEPEFKQEIIGLAEVRDVFRHPKFGAIAGCMVTEGVVKRNNPIRVLRDNVVIFEGELESLRRFKDDVSEVRNGMECGIGVKNYNDVKVGDQIEVFEVVEVKRSI |
| Purity : | >85% |
| Storage : | Short Term Storage at 4 centigrade, Long Term, please prepare aliquots with 20% glycerol and store at -20 to -80 centigrade. Avoid freeze/thaw cycles. |
| Concentration : | 1.0 mg/ml |
| Gene Name | infB translation initiation factor IF-2 [ Haemophilus influenzae ] |
| Official Symbol | infB |
| Gene ID | 72525378 |
| Protein Refseq | WP_044330186 |
| UniProt ID | Q4QK37 |
| ◆ Recombinant Proteins | ||
| INFB-1047S | Recombinant Streptomyces coelicolor A3(2) INFB protein, His-tagged | +Inquiry |
| INFB-2828S | Recombinant Staphylococcus epidermidis ATCC 12228 INFB protein, His-tagged | +Inquiry |
| infB-40M | Recombinant Moraxella catarrhalis infB Protein | +Inquiry |
| INFB-0718B | Recombinant Bacillus subtilis INFB protein, His-tagged | +Inquiry |
| infB-112H | Recombinant Haemophilus influenzae infB Protein | +Inquiry |
Case 1: Eiler D, et al. Proc Natl Acad Sci U S A. 2013
The initiation of protein synthesis uses initiation factor 2 (IF2) in prokaryotes and a related protein named eukaryotic initiation factor 5B (eIF5B) in eukaryotes. IF2 is a GTPase that positions the initiator tRNA on the 30S ribosomal initiation complex and stimulates its assembly to the 50S ribosomal subunit to make the 70S ribosome. The 3.1-Å resolution X-ray crystal structures of the full-length Thermus thermophilus apo IF2 and its complex with GDP presented here exhibit two different conformations (all of its domains except C2 domain are visible). Unlike all other translational GTPases, IF2 does not have an effecter domain that stably contacts the switch II region of the GTPase domain. The domain organization of IF2 is inconsistent with the "articulated lever" mechanism of communication between the GTPase and initiator tRNA binding domains that has been proposed for eIF5B. Previous cryo-electron microscopy reconstructions, NMR experiments, and this structure show that IF2 transitions from being flexible in solution to an extended conformation when interacting with ribosomal complexes.
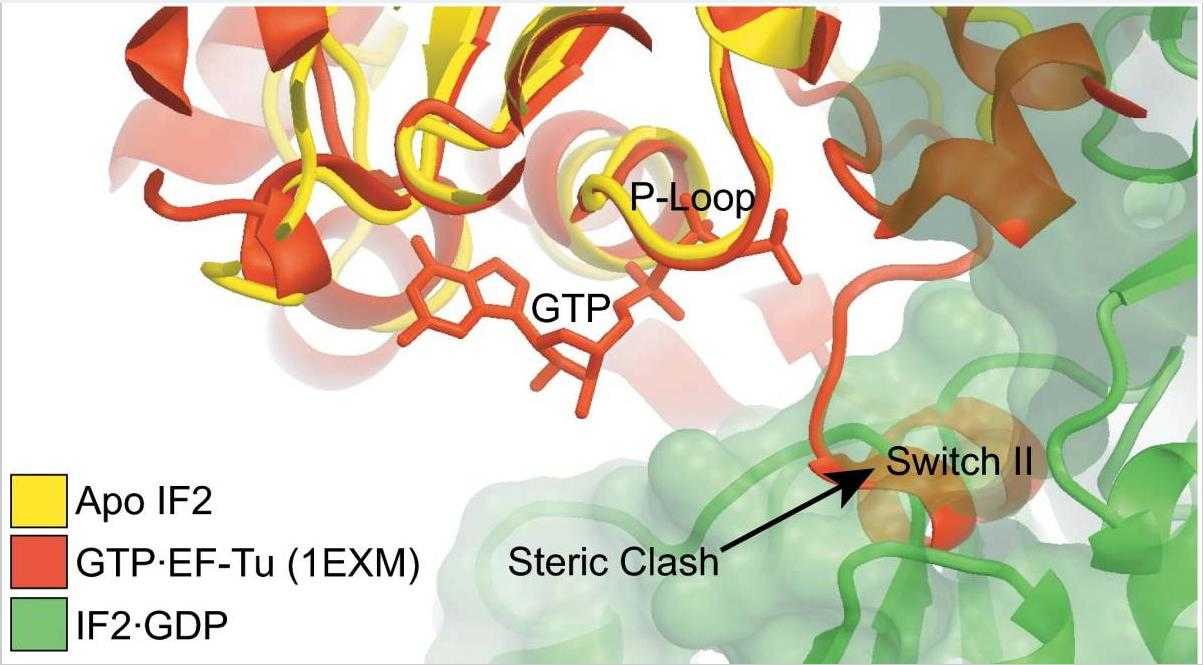
Fig1. G-nucleotide binding pocket of Apo IF2.
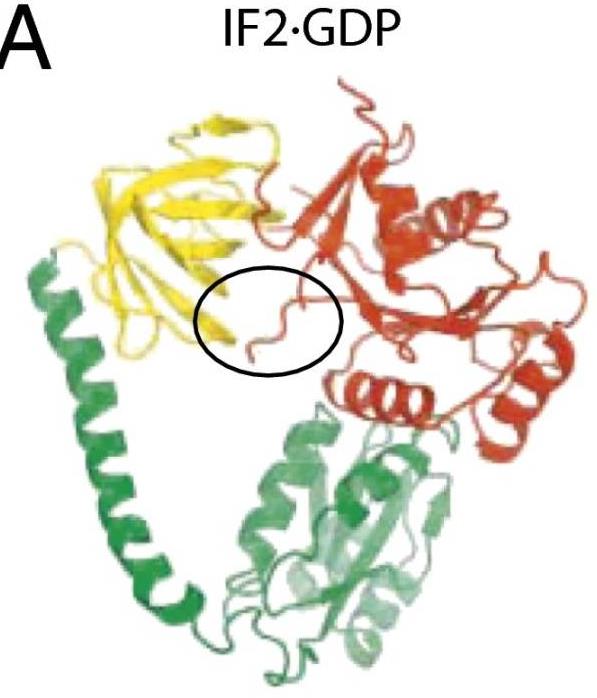
Fig2. IF2 with GDP bound.
Case 2: Caban K, et al. Nat Commun. 2017
Initiation factor (IF) 2 controls the fidelity of translation initiation by selectively increasing the rate of 50S ribosomal subunit joining to 30S initiation complexes (ICs) that carry an N-formyl-methionyl-tRNA (fMet-tRNAfMet). Previous studies suggest that rapid 50S subunit joining involves a GTP- and fMet-tRNAfMet-dependent "activation" of IF2, but a lack of data on the structure and conformational dynamics of 30S IC-bound IF2 has precluded a mechanistic understanding of this process. Here, using an IF2-tRNA single-molecule fluorescence resonance energy transfer signal, researchers directly observe the conformational switch that is associated with IF2 activation within 30S ICs that lack IF3. Based on these results, we propose a model of IF2 activation that reveals how GTP, fMet-tRNAfMet, and specific structural elements of IF2 drive and regulate this conformational switch.
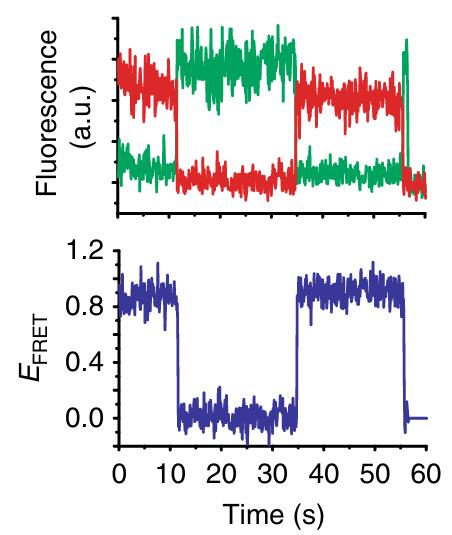
Fig1. smFRET measurements of wtIF2(GTP) with 30S ICwT.
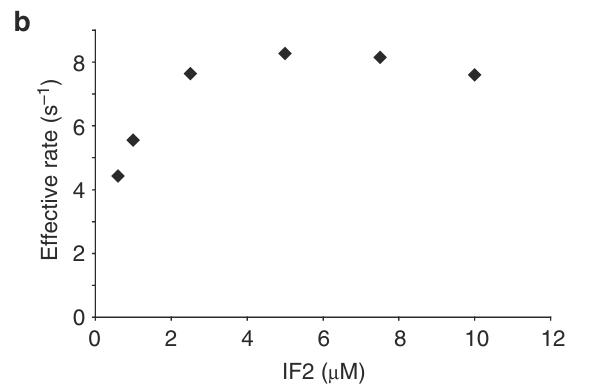
Fig2. Effective rates of 50S subunit joining to 30S ICwT,OHs containing increasing concentrations of wtIF2.
The homologous protein of initiation factor 2 (IF-2) in Escherichia coli is a key component in bacterial protein synthesis, responsible for promoting the binding of 30S ribosomal subunits to mRNA and the accurate placement of initiation trnas. In Haemophilus influenzae, the function and application of IF-2 may be similar to that in E. coli, but the specific application scenarios may be different.
1. Antibiotic development. Studies have shown that specific domains of IF-2, especially domain III, play a key allosteric role in the activation of IF-2, which provides a possible target for the development of novel antibiotics.
2. Pathogen research and treatment: Because H. influenzae is an important human pathogen, studying the function of its IF-2 may help develop specific treatment strategies against this bacterium.
3. Study on gene expression regulation. In studying the assembly of the 30S initiation complex (IC), the interaction of IF-2 with tRNA as well as mRNA is key to understanding the initiation of the translation process. By studying the function of IF-2, strategies can be explored to improve translation efficiency, for example by modifying the IF-2 protein or its interaction with the ribosome.
4. Structural biology research. Using single-molecule fluorescence resonance energy transfer (smFRET) techniques, researchers can observe conformational changes associated with IF-2 activation, and high-resolution structural studies of IF-2 help reveal its mechanism of action during translation, providing a structural basis for understanding protein-RNA interactions.
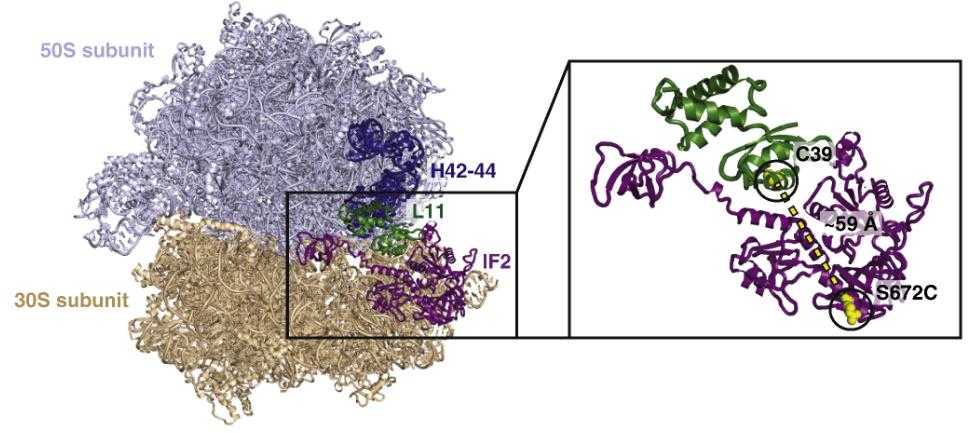
Fig1. Monitoring subunit joining by smFRET between L11 and IF2. (Daniel D MacDougall, 2015)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All infB Products
Required fields are marked with *
My Review for All infB Products
Required fields are marked with *
Inquiry Basket


