Recombinant Chicken FAP Protein, K30-E759, N-His tagged
| Cat.No. : | FAP-4123C |
| Product Overview : | Recombinant Chicken FAP protein (K30-E759) with N-His tag was expressed in HEK293. |
| Availability | April 19, 2025 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Chicken |
| Source : | HEK293 |
| Tag : | His |
| Protein Length : | K30-E759 |
| Description : | The protein encoded by this gene is a homodimeric integral membrane gelatinase belonging to the serine protease family. It is selectively expressed in reactive stromal fibroblasts of epithelial cancers, granulation tissue of healing wounds, and malignant cells of bone and soft tissue sarcomas. This protein is thought to be involved in the control of fibroblast growth or epithelial-mesenchymal interactions during development, tissue repair, and epithelial carcinogenesis. Alternatively spliced transcript variants encoding different isoforms have been found for this gene. |
| Molecular Mass : | 85 kDa |
| AA Sequence : | HHHHHHHHKVVTTVDGPRALTLDDYLNGNFQYKTYFPYWVSDSEYLHQNQEDDIILFNVDMNYLTTIMTNSTMKQVNASNYVMSSDKYFIALESNYSKLWRYSYTASYHIYDLIYGGFVTENQLPHKIQYISWSPVGHKLAYVYQNNIYLKQSPREAPIKLTSDGKENEIFNGIPDWVYEEEMLATKYALWWSPSGKYLAYVQFNDSDIPVIEYSYFGEDQYPRKIIIPYPKAGAKNPTVKVFIVDTTNIEAFGPKEVPVPAVIASSDHYFTWLTWVTDSRVGVQWLKRIQNFSVLAICDFKENSNTWDCPENQQHIEESQTGWAGGFFVSAPYFTSDGSSYYKIFSDKNGYKHIHYINGSVENAIQITSGEWEAIYIFRVTNDAIFYSSNEFEGYPGRRNIYKISIGSKPIRKLCITCNLRKERCQYYTARFSERSKYYALICYGPGIPISTLFENESDRELRILEDNQELQSALQEIILPKEEINKLEVDGITLWYKMLIPPQFDRSKKYPLLIQVYGGPCSQNVKHTFSISWITYLASKEGIIVALVDGRGTAYQGDKILHAVYRRLGVYEVEDQISAVKKFIEMGFIDEKRIAIWGWSYGGYVTSLALGSGSGVFKCGIAVAPVSSWEYYASIYTERFMGLPVESDNLEHYKNSTVMARAKNFQNVEYLLIHGTADDNVHFQNSAQIAKALVNAQVDFQAMWYTDQNHGIPGLSSKHLYTHMTHFLKQCFSLSE |
| Endotoxin : | < 1 EU/μg by LAL |
| Purity : | > 95% by SDS-PAGE |
| Storage : | Store it under sterile conditions at -20 to -80 centigade. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Concentration : | 0.38 mg/mL by BCA |
| Storage Buffer : | Sterile PBS, pH7.4 |
| Gene Name | FAP fibroblast activation protein alpha [ Gallus gallus (chicken) ] |
| Official Symbol | FAP |
| Synonyms | FAP; fibroblast activation protein alpha; prolyl endopeptidase FAP; EC 3.4.14.5; EC 3.4.21.26 |
| Gene ID | 424186 |
| mRNA Refseq | NM_001193639 |
| Protein Refseq | NP_001180568 |
| UniProt ID | A0A8V0ZGU9 |
| ◆ Recombinant Proteins | ||
| FAP-426H | Recombinant Human Fibroblast Activation Protein, Alpha, His-tagged | +Inquiry |
| Fap-2373M | Recombinant Mouse Fap protein, His-SUMO-tagged | +Inquiry |
| FAP-006H | Active Recombinant Human FAP Protein, His-tagged | +Inquiry |
| Fap-1018M | Recombinant Mouse Fap Protein, MYC/DDK-tagged | +Inquiry |
| FAP-370CAF488 | Active Recombinant Monkeys FAP Protein, His-tagged, Alexa Fluor 488 conjugated | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| FAP-1881HCL | Recombinant Human FAP cell lysate | +Inquiry |
Case 1: Dohmen RGJ, et al. NPJ Sci Food. 2022
Cultured meat technology offers environmental and animal welfare advantages, and the replication of traditional meat's taste and texture relies on fat tissue. The research reveals that fibro-adipogenic progenitor cells (FAPs) in bovine muscle, which are distinct from satellite cells, can be isolated using a straightforward fluorescence-activated cell sorting (FACS) method. These FAPs exhibit substantial adipogenic potential, as indicated by gene expression and lipid accumulation, and can be expanded for numerous population doublings, making them suitable for large-scale cultured meat production. Importantly, FAPs mature into adipogenic differentiation within three-dimensional, edible hydrogels, producing tissue that closely resembles traditional beef fat in lipid composition and taste, marking them as a promising cell type for cultured fat production.
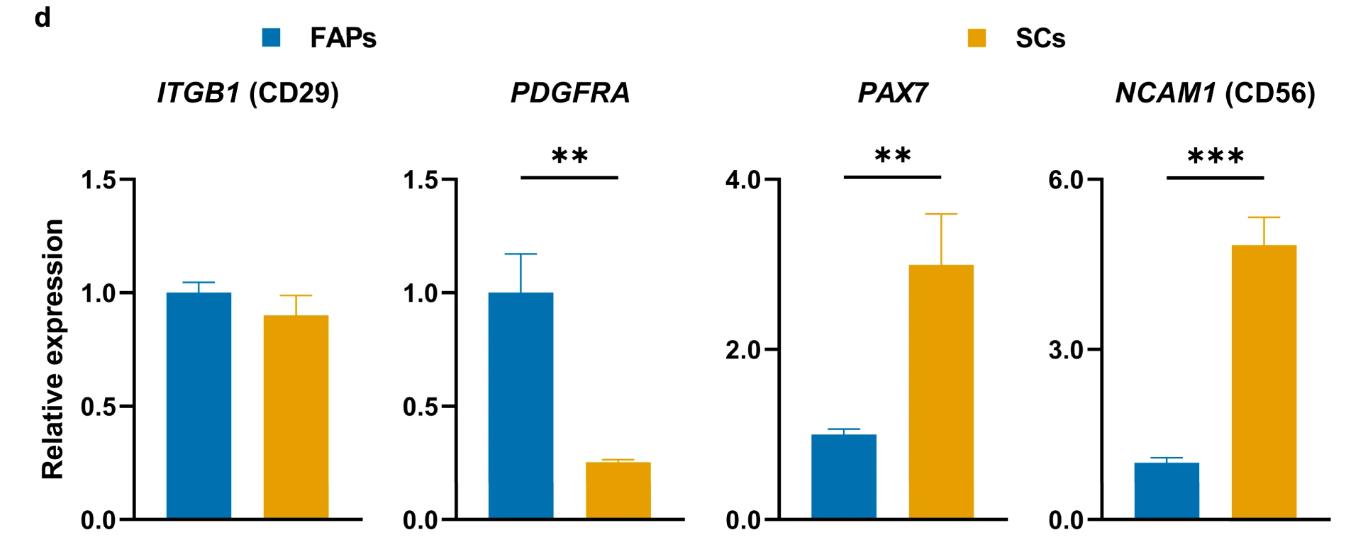
Fig1. Expression of selected stem cell marker genes in FAPs and SCs after sorting, as measured by RT-qPCR.
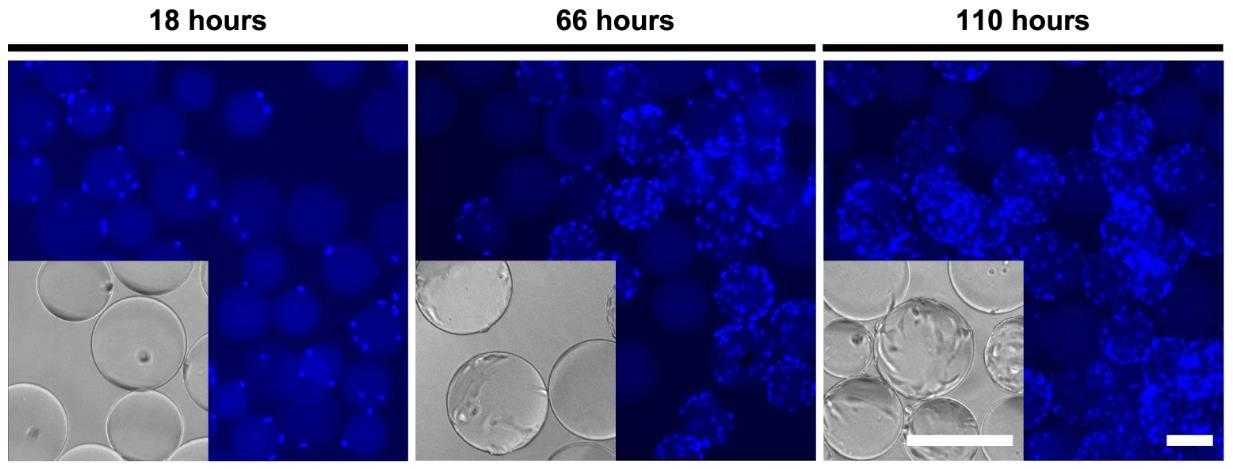
Fig2. Representative brightfield and immunofluorescence microscopy images of FAPs cultured on Cytodex 1 microcarriers.
Case 2: Wang Y, et al. EJNMMI Res. 2024
Assessing skeletal muscle injuries is complex, and this study investigated the utility of 68Ga-FAPI-04 for monitoring muscle remodeling. In vitro, C2C12 cells showed variable FAP expression with increasing H2O2 levels. In a murine muscle injury model mimicking different repair outcomes, PET imaging with 68Ga-FAPI-04 on day 1 distinguished between normal and mild repair failure groups, which had higher SUVmax and T/B ratios, and a severe repair failure group with lower values. These ratios decreased over time in the normal repair group, becoming minimal by day 7, correlating with MRI signal intensity changes. Retrospective clinical analysis of hip arthroplasty patients showed stable 18F-FDG SUVmax and volume over months, while 68Ga-FAPI-04 SUVmax increased initially then decreased, with a consistent volume reduction, indicating its potential for tracking muscle repair.
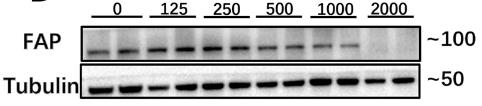
Fig1. Western blot analysis performed to study the expression levels of FAP in C2C12-induce myofiber.
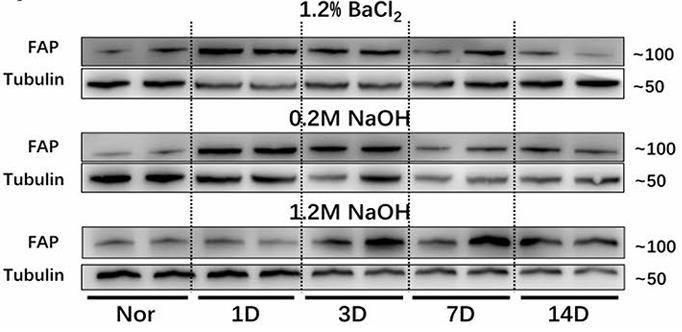
Fig2. Western blot analysis performed to study the expression levels of FAP in different models.
Recombinant chicken FAP protein has important value in scientific research and clinical applications. FAP (fibroblast activation protein) is an enzyme associated with extracellular matrix remodeling and wound healing. It is highly expressed in a variety of pathological conditions such as cancer and fibrotic diseases, making it a potential marker and therapeutic target for studying tumor microenvironment and fibrosis. In cancer, FAP is mainly expressed in tumor-associated fibroblasts and is involved in tumor growth and metastasis, so understanding the function of FAP can provide important clues for the diagnosis and treatment of the disease.
In scientific research applications, recombinant FAP protein is an important tool for studying the tumor microenvironment, especially in exploring the role of FAP in tumor formation, progression and metastasis. It is also used to study the development and regulatory mechanisms of fibrosis. In the biotechnology and pharmaceutical fields, recombinant FAP protein is used for drug screening to discover compounds that can inhibit or regulate FAP activity, thereby developing anti-cancer or anti-fibrosis drugs. In addition, the potential of FAP as a therapeutic target has triggered the development of new biologics, and recombinant proteins can be used to design innovative therapies for specific pathological conditions. In the development of diagnostic tools, recombinant FAP protein can be used to develop diagnostic tools to detect FAP expression levels to help identify and monitor related diseases.
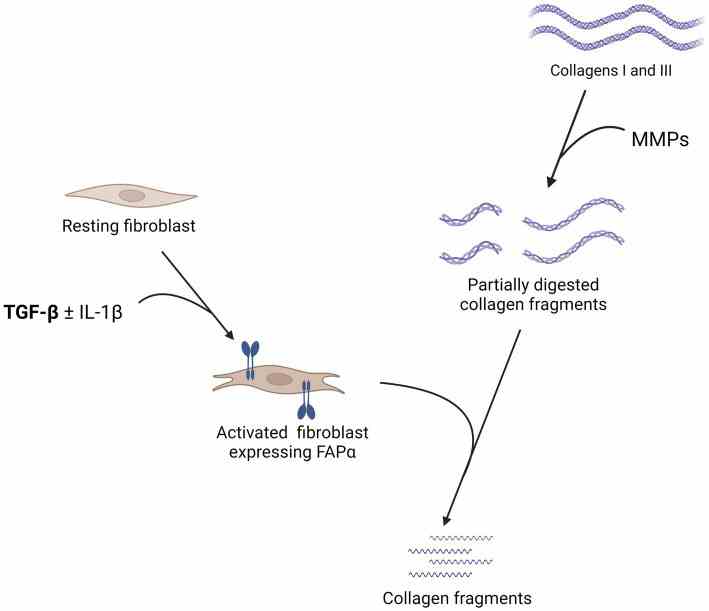
Fig1. Summary of the pathophysiology of the fibroblast activation protein α (FAPα) in lung fibrosis. (Philomène Lavis, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All FAP Products
Required fields are marked with *
My Review for All FAP Products
Required fields are marked with *
Inquiry Basket


