Recombinant Canine FGF12 protein(Glu2-Thr181)
| Cat.No. : | FGF12-434C |
| Product Overview : | Recombinant canine FGF12 (Glu2-Thr181) was expressed in E. coli with a N-terminal Met. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Canine |
| Source : | E.coli |
| Tag : | Non |
| Protein Length : | 2-181 a.a. |
| Form : | Lyophilized from sterile PBS, pH 7.4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant canine FGF12 comprises 181 amino acids and has a predicted molecular mass of 20.4 kDa. The apparent molecular mass of the protein is approximately 20 kDa in SDS-PAGE under reducing conditions due to glycosylation. |
| Purity : | > 90 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | FGF12 fibroblast growth factor 12 [ Canis lupus familiaris (dog) ] |
| Official Symbol | FGF12 |
| Synonyms | FGF12; fibroblast growth factor 12 |
| Gene ID | 478676 |
| mRNA Refseq | XM_003434129 |
| Protein Refseq | XP_003434177 |
| ◆ Recombinant Proteins | ||
| FGF12-4809HF | Recombinant Full Length Human FGF12 Protein, GST-tagged | +Inquiry |
| FGF12-105H | Active Recombinant Human FGF12 Protein (Met1-Thr181), C-His tagged, Animal-free, Carrier-free | +Inquiry |
| FGF12-026H | Active Recombinant Human FGF12 Protein | +Inquiry |
| FGF12-2325R | Recombinant Rat FGF12 Protein | +Inquiry |
| FGF12-1982R | Recombinant Rat FGF12 Protein, His (Fc)-Avi-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| FGF12-6249HCL | Recombinant Human FGF12 293 Cell Lysate | +Inquiry |
Case 1: Biadun M, et al. Cell Mol Life Sci. 2024
FGF12 is part of the FGF homologous factors (FHFs) subfamily, previously considered to be non-signaling, intracellular FGF proteins. However, this research has demonstrated that these proteins, despite lacking a traditional secretory signal peptide, can be released into the extracellular environment, particularly under stress. Here the long "a" isoform of FGF12 is secreted via a pathway that includes the A1 subunit of Na(+)/K(+) ATPase (ATP1A1), Tec kinase, and lipids like phosphatidylinositol and phosphatidylserine. In contrast, the short "b" isoform, w hich interacts less effectively with ATP1A1 and phosphatidylserine, does not get secreted. Researchers have identified key regions within the FGF12a sequence necessary for its secretion, such as the N-terminal fragment and specific residues, and suggest that liquid-liquid phase separation could play a significant role in this process.
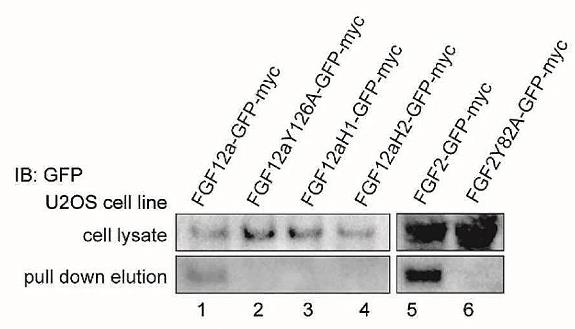
Fig1. The eluted proteins and lysates were analyzed by SDS-PAGE and western blotting using anti-GFP antibody.
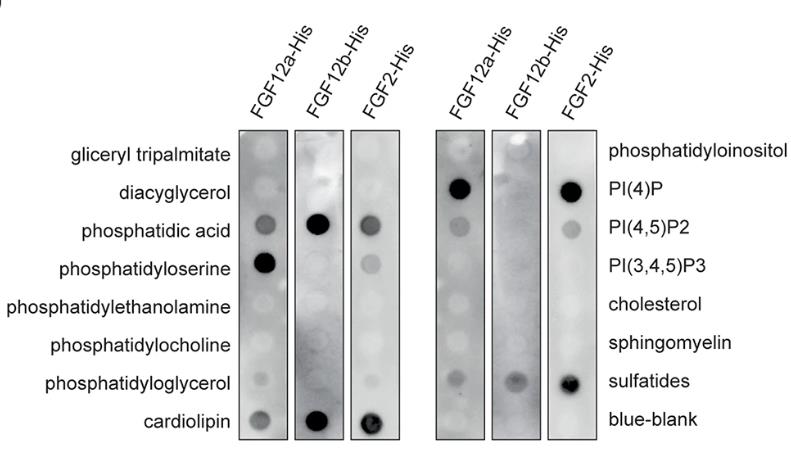
Fig2. Membrane lipid strips were incubated with FGF2-His, FGF12a-His and FGF12b-His.
Case 2: Sochacka M, et al. Cell Commun Signal. 2022
Within the FGF protein family, the fibroblast growth factor homologous factors (FHFs) are the least understood. Traditionally, FHFs are known for their role in modulating voltage-gated ion channels, but their intracellular functions remain largely unclear. This study aimed to uncover new FGF12 binding partners to shed light on its cellular roles. Here many of these partners are nuclear proteins, particularly RNA-binding proteins that play a role in translational processes such as ribosomal processing and modification. FGF12 was found to be localized in the nucleolus, where it interacts with NOLC1 and TCOF1, which are crucial for ribosome assembly. These interactions are unique to FGF12, as other FHFs only interact with TCOF1. The nucleolar complexes formed by FGF12 with NOLC1 and TCOF1 depend on phosphorylation and the C-terminal region of FGF12. Interestingly, NOLC1 and TCOF1 do not interact in the absence of FGF12.
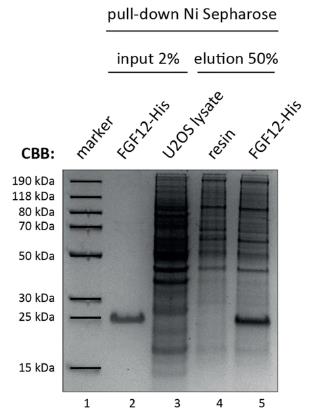
Fig1. Recombinant FGF12-His was bound to Ni Sepharose and incubated with U2OS cell lysate.
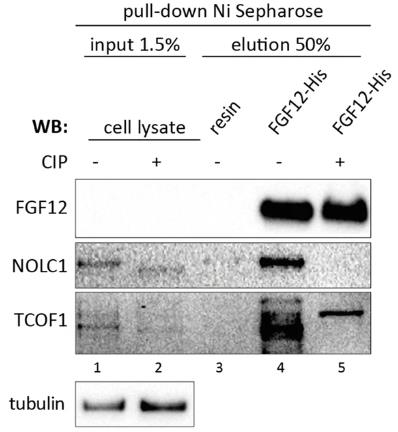
Fig2. Pull-down experiment with recombinant FGF12-His bound to Ni Sepharose and U2OS cell lysate.
Recombinant canine FGF12 protein has important application value in scientific research and preclinical studies. FGF12 is a member of the fibroblast growth factor family, which plays a key role in a variety of biological processes, including cell growth, development, wound healing and tissue repair. In the nervous system, in particular, FGF12 is closely related to cell survival and differentiation processes.
In the field of scientific research, recombinant FGF12 protein is widely used in neuroscience research to explore the growth, differentiation and survival mechanisms of nerve cells. In addition, it also plays an important role in developmental biology, helping scientists better understand the complex interactions of signaling pathways during development. In preclinical studies, recombinant FGF12 protein is used in drug development by screening and testing compounds that may affect FGF12-related pathways, as well as mimicking human diseases in animal models to study pathology and possible treatments. In biotechnology and industrial applications, recombinant FGF12 protein is used as a tool protein for designing and testing novel protein structures, as well as promoting tissue repair and regeneration processes in tissue engineering.
In addition, FGF12 is associated with neurological diseases, cancer and heart disease, making it a potential target for gene therapy and therapeutic agents. Studies have shown that the expression of FGF12 is positively correlated with the degree of liver fibrosis, which indicates that it may play a promoting role in liver fibrosis. Therefore, inhibitors targeting FGF12 may become a potential strategy for the treatment of liver fibrosis. These applications not only promote further understanding of the function and mechanism of FGF12, but also provide valuable tools for new targets for the future treatment of various diseases.
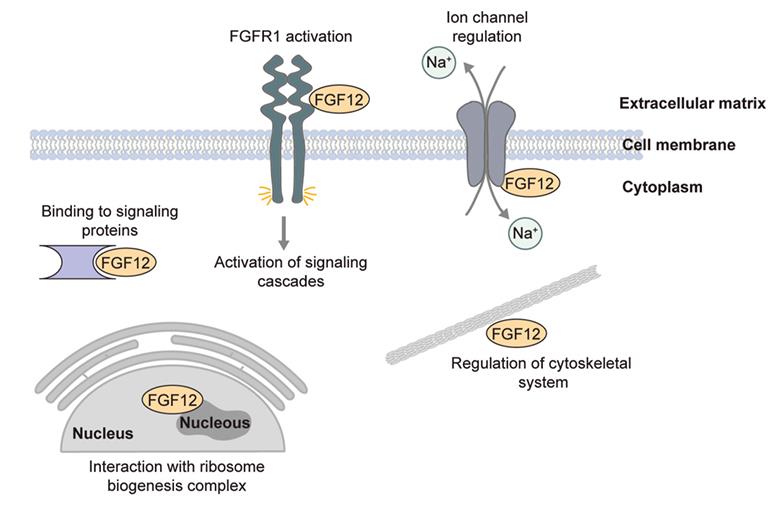
Fig1. Functions of FGF12 in the cell. (Martyna Biadun, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All FGF12 Products
Required fields are marked with *
My Review for All FGF12 Products
Required fields are marked with *
Inquiry Basket


