Native Human Cancer Antigen 72-4
| Cat.No. : | CDA007 |
| Product Overview : | Human Cancer Antigen 72-4 purified from metastatic Liver Carcinoma. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | Human Metastatic Liver Carcinoma |
| Tag : | Non |
| Description : | Elevated CA 72-4 levels in serum and plasma have been reported in various malignant diseases including carcinomas of pancreas, stomach, gallbladder, colon, ovaries, cervix and endometrium. The highest diagnostic sensitivities, according to current studies, are found for carcinomas of the gastrointestinal tract and ovaries. Although some benign diseases such as rheumatic diseases or ovary cysts may also result in elevated levels of CA 72-4, clinical studies demonstrated diagnostic specificity of more than 95% for gastrointestinal and ovarian malignancies. There is a good correlation between CA 72-4 levels and tumor stage and size. CA 72-4 is the marker of choice for the therapeutic monitoring and follow-up care of gastrointestinal cancer patients. Suitable second markers are CA 19-9 or CEA. It has been used as an independent marker for the therapeutic monitoring and follow-up care of ovarian cancer patients, in particular in CA 125 negative patients. |
| Tag : | Non |
| Form : | Liquid |
| Bio-activity : | ≥ 100 kU/mL |
| Molecular Mass : | > 1,000,000 |
| Purity : | > 90% by SDS-PAGE |
| Applications : | Immunoassay/Immunology, Tumor/Cancer |
| Storage : | At -20 centigrade. |
| Storage Buffer : | Solution in phosphate buffered saline, pH 7.4 with sucrose and 0.05% sodium azide. |
| Official Symbol | CA 72-4 |
| Synonyms | CA 72-4, Cancer Antigen 72-4, Cancer Antigen Tag-72, Cancer Antigen 72-4, Cancer Antigen Tag-72 |
| ◆ Native Proteins | ||
| CDA007 | Native Human Cancer Antigen 72-4 | +Inquiry |
Case 1: Chen Y, et al. Anal Bioanal Chem. 2016
A sensitive and specific quantitative lateral flow immunoassay (LFIA) using superparamagnetic nanoparticles (SPMNPs) has been developed for the detection of the cancer biomarker CA72-4 in human serum. This method requires no sample preparation and employs a test strip for a rapid sandwich reaction. The test strip features monoclonal antibody CC49 conjugated to SPMNPs and a nitrocellulose membrane with test and control lines consisting of monoclonal antibody B72.3 and goat anti-mouse IgG. The assay's magnetic signal is quantified using a magnetic assay reader (MAR), and the optimized protocol enables the detection of CA72-4 at concentrations as low as 0.38 IU/mL within 20 minutes, with a broad linear range of 0-100 IU/mL. Evaluation of 100 clinical samples demonstrated the test strip's high sensitivity (99%), specificity (97%), and accuracy (98.4-102%), making it a promising tool for cancer diagnosis and monitoring.
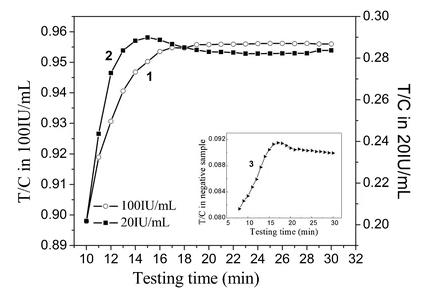
Fig1. Plot of T/C ratio versus testing time.
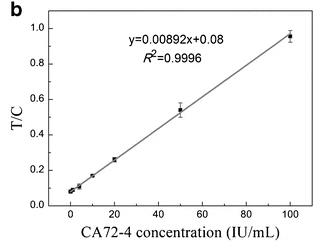
Fig2. Calibration curve for quantitative detection of CA72-4.
Case 2: Evtimov VJ, et al. Heliyon. 2024
Monoclonal antibody therapies for cutaneous T cell lymphoma (CTCL) require specific tumor targets absent in normal cells. We suggest tumor associated glycoprotein-72 (TAG-72) as a potential target, already identified in solid tumors for CAR-T cell therapy. This study investigates TAG-72 expression in CTCL patients' T cells and skin lesions, and soluble TAG-72 (CA 72-4) in plasma. TAG-72 CAR-T cells were generated and tested for function against CTCL cells in vitro and in a TAG-72+ ovarian cancer mouse model.
TAG-72 was more highly expressed in CTCL T cells than in healthy donors. It was also found in CTCL skin lesions, while CA 72-4 levels were similar in CTCL and healthy plasma. Anti-TAG-72 CAR-T cells effectively targeted CTCL cells in vitro, and CTCL-derived CAR-T cells showed comparable functionality to healthy donor cells, eliminating cancer cells in vivo. These findings support TAG-72 as a viable target for CTCL treatment.
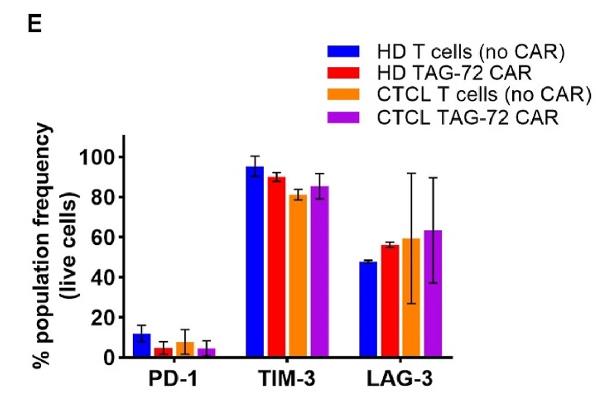
Fig1. Exhaustion markers PD-1, TIM-3 and LAG-3 in CAR- and T cell (no CAR) controls, and TAG-72+ cells remaining in the CD8+ CAR-T cell product.
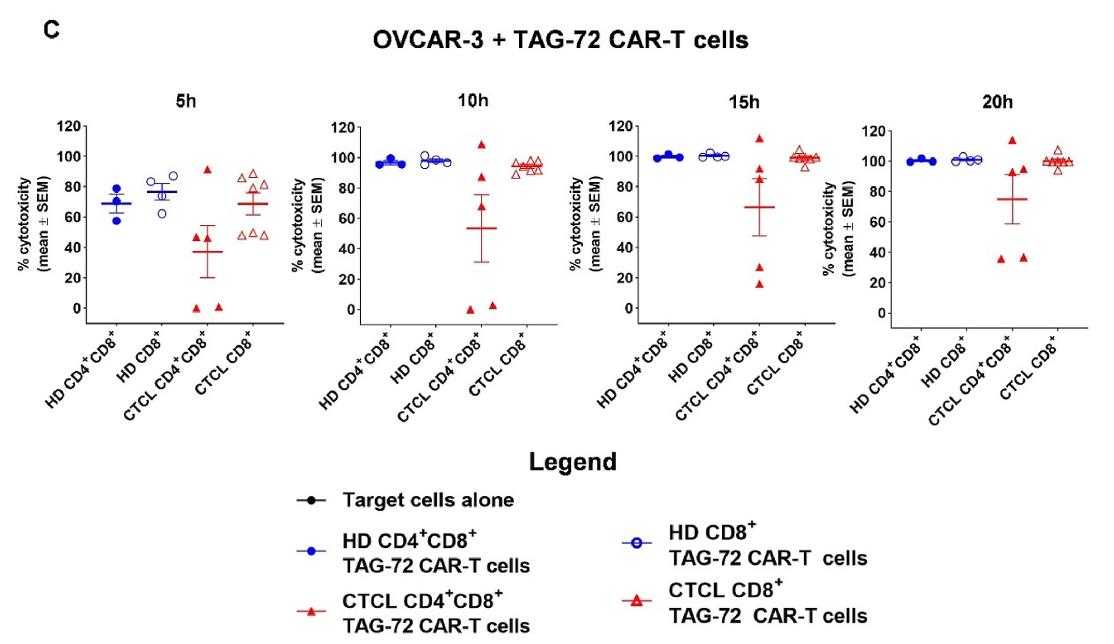
Fig2. Efficiency of target cell killing was quantitated at 5–20 h (h) of OVCAR-3 and TAG-72 CAR-T cell co-culture and is presented as mean percent cytotoxicity.
Cancer Antigen 72-4 (CA 72-4) is a tumor marker that is primarily associated with gastric and ovarian cancer, although it can also be linked to various adenocarcinomas, particularly those in the gastrointestinal tract, ovaries, and breast. This mucin-like glycoprotein complex is found in fetal tissue and is measured in the blood to indicate the presence of malignancies, with elevated levels suggesting cancer.
In daily life and research, CA 72-4 serves several applications. Diagnostically, it is used for screening and monitoring gastrointestinal cancers, especially gastric cancer. Its levels can be utilized to assess treatment response and detect recurrence. Often, CA 72-4 is measured alongside other tumor markers like Carcinoembryonic Antigen (CEA) and CA 19-9, to enhance the accuracy of diagnosing gastrointestinal cancers. In research, CA 72-4 is a subject of interest to understand tumor biology and develop targeted therapies. Its effectiveness and utility in cancer detection and management are major areas of clinical trial investigations. For therapeutic monitoring, variations in CA 72-4 levels assist physicians in gauging the efficacy of ongoing treatments, including chemotherapy and surgical interventions. Prognostically, high levels of CA 72-4 at diagnosis can indicate a poor prognosis, providing vital information for devising a management plan. Furthermore, CA 72-4 is used in the follow-up of patients with malignant tumors to monitor disease progression and recurrence, and to assess the effectiveness of treatments. It is also used in clinical trials to explore new diagnostic and therapeutic strategies, and as a research tool to better understand the biology of various cancers.
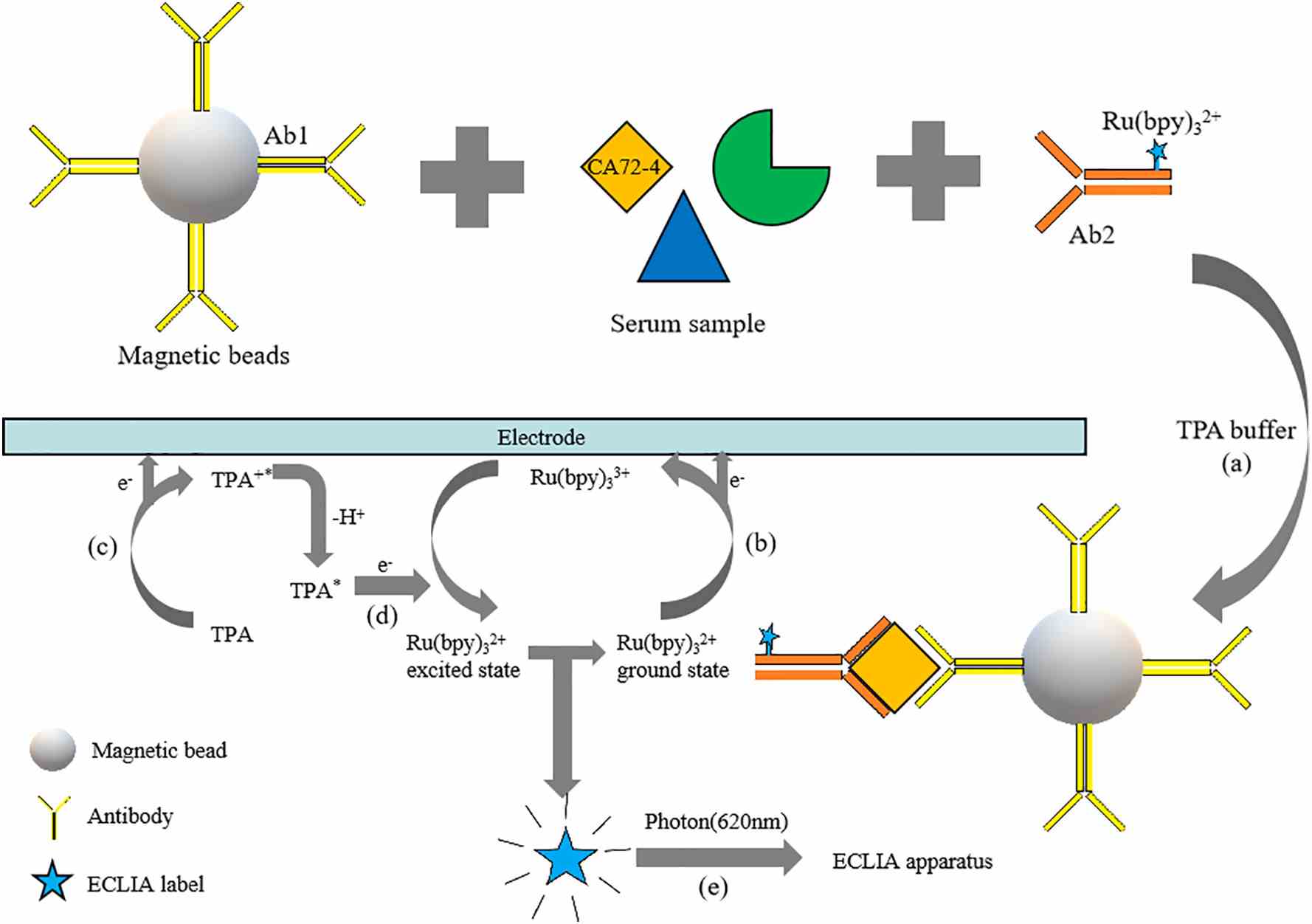
Fig1. Principle of electrochemiluminescence immunoassay of serum CA72-4. (Yitian Xu, 2021)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All CA 72-4 Products
Required fields are marked with *
My Review for All CA 72-4 Products
Required fields are marked with *
Inquiry Basket


