Active Recombinant Human PYCR1 protein, His-tagged, Bioactivity Validated.
| Cat.No. : | PYCR1-31271TH |
| Product Overview : | Recombinant Full Length Human PYCR1(1-319aa) fused with His tag at N-terminal was expressed in E. coli. |
| Availability | April 19, 2025 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | 1-319 a.a. |
| Description : | PYCR1 is a universal housekeeping enzyme that catalyzes the NAD(P)H-dependent conversion of pyrroline-5-carboxylate to proline. This enzyme may also play a physiologic role in the generation of NADP(+) in some cell types. It forms a homopolymer and localizes to the mitochondrion. Defects in PYCR1 are the cause of cutis laxa autosomal recessive type 2B (ARCL2B). |
| Form : | Liquid. In 20 mM Tris-HCl Buffer (pH 8.5) containing 10% Glycerol |
| Bio-activity : | Specific activity is > 0.03 unit/mg. One unit will oxidize 1.0 umole of L-proline to 1-pyrroline-5-carboxylate per minute in the presence of beta NAD at pH 10.0 at 25C. |
| Molecular Mass : | 35.5 kDa(339aa), confirmed by MALDI-TOF |
| Purity : | > 90% by SDS - PAGE |
| Storage : | Can be stored at +4 centigrade short term (1-2 weeks). For long term storage, aliquot and store at -20 centigrade or -70 centigrade. Avoid repeated freezing and thawing cycles. |
| Concentration : | 0.5 mg/ml (determined by Bradford assay) |
| Publications : |
(S)-4-Amino-5-phenoxypentanoate designed as a potential selective agonist of the bacterial transcription factor GabR (2020)
|
| Gene Name | PYCR1 pyrroline-5-carboxylate reductase 1 [ Homo sapiens ] |
| Official Symbol | PYCR1 |
| Synonyms | PYCR1; pyrroline-5-carboxylate reductase 1; pyrroline-5-carboxylate reductase 1, mitochondrial; P5C; P5CR 1; P5C reductase 1; proliferation-inducing protein 45; mitochondrial pyrroline-5-carboxylate reductase 1; P5CR; PRO3; PYCR; PIG45; PP222; ARCL2B; ARCL3B; |
| Gene ID | 5831 |
| mRNA Refseq | NM_006907 |
| Protein Refseq | NP_008838 |
| MIM | 179035 |
| UniProt ID | P32322 |
| Chromosome Location | 17 |
| Pathway | Amino acid synthesis and interconversion (transamination), organism-specific biosystem; Arginine and proline metabolism, organism-specific biosystem; Arginine and proline metabolism, conserved biosystem; Metabolic pathways, organism-specific biosystem; Metabolism, organism-specific biosystem; Metabolism of amino acids and derivatives, organism-specific biosystem; Proline biosynthesis, glutamate => |
| Function | identical protein binding; nucleotide binding; oxidoreductase activity; oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor; pyrroline-5-carboxylate reductase activity; pyrroline-5-carboxylate reductase activity; |
| ◆ Recombinant Proteins | ||
| PYCR1-31271TH | Active Recombinant Human PYCR1 protein, His-tagged, Bioactivity Validated. | +Inquiry |
| PYCR1-1815H | Recombinant Human PYCR1 Protein, His (Fc)-Avi-tagged | +Inquiry |
| PYCR1-3536R | Recombinant Rhesus Macaque PYCR1 Protein, His (Fc)-Avi-tagged | +Inquiry |
| PYCR1-6125H | Recombinant Human PYCR1 Protein (Ser2-Asp319), N-His tagged | +Inquiry |
| PYCR1-3719R | Recombinant Rhesus monkey PYCR1 Protein, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| PYCR1-1448HCL | Recombinant Human PYCR1 cell lysate | +Inquiry |
Case 1: Catlin DS, et al. Protein Sci. 2020
Addressing molecular recognition in the context of evolution requires pursuing new molecular targets to enable the development of agonists or antagonists with new mechanisms of action. Disruption of transcriptional regulation through targeting transcription factors that regulate the expression of key enzymes in bacterial metabolism may provide a promising method for controlling the bacterial metabolic pathways. To this end, researchers have selectively targeted a bacterial transcription regulator through the design and synthesis of a series of γ-aminobutyric acid (GABA) derivatives, including (S)-4-amino-5-phenoxypentanoate (4-phenoxymethyl-GABA).
In this study, they have obtained a crystal structure of 4-phenoxymethyl-GABA bound as an external aldimine with PLP in the effector binding site of GabR. While rhPYCR1 protein was used to verify the role of PLP as a coenzyme.
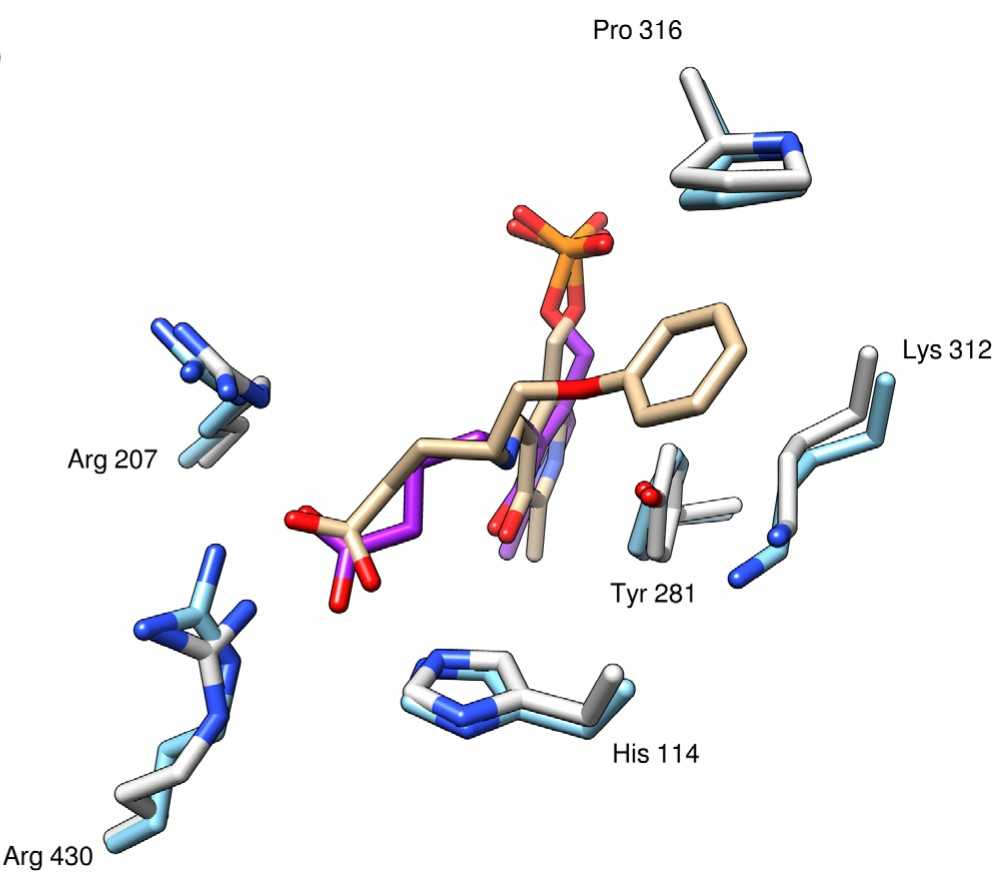
Fig1. A superimposition of the external aldimine PLP-GABA in magenta (PDB code: 5T4J) and the external aldimine PGP in tan with surrounding residues in the binding site.
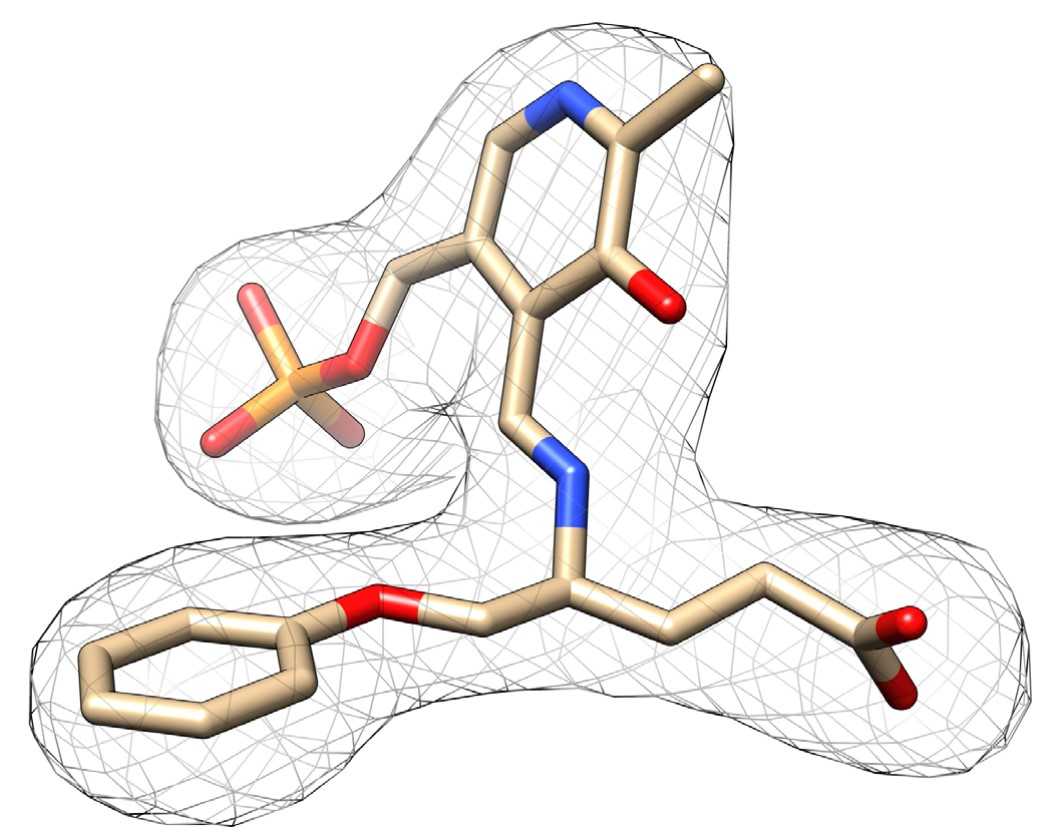
Fig2. A polder map (F o -F c) of PLP-13 (PGP).
Case 2: Li Z, et al. Int J Oncol. 2023
Bladder cancer (BC) is a heterogeneous disease, and pyrroline‑5‑carboxylate reductase 1 (PYCR1) can promote the proliferation and invasion of BC cells and accelerate BC progression. In the present study, si‑PYCR1 was loaded into bone marrow mesenchymal stem cell (BMSC)‑derived exosomes (Exos) in BC. PYCR1‑EGFR interactions were examined by co‑immunoprecipitation experiments. Exos were loaded with si‑PYCR1 and identified, followed by an assessment of their effects on aerobic glycolysis and malignant cell behaviors. The results revealed Exo‑si‑PYCR1 blocked xenograft tumor growth and had good biocompatibility.
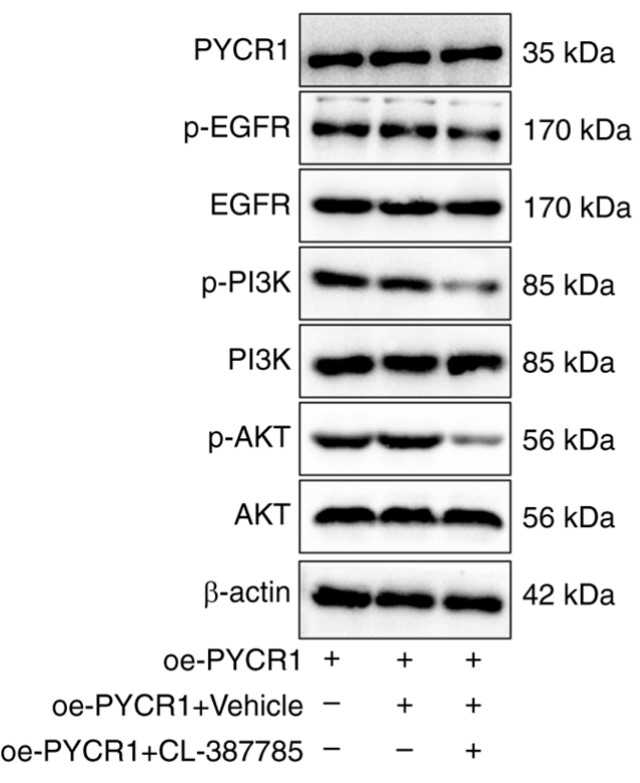
Fig1. Western blot found no change in protein expression of PYCR1 and repressed phosphorylation levels of the EGFR/PI3K/AKT pathway-related proteins in RT4 cells.
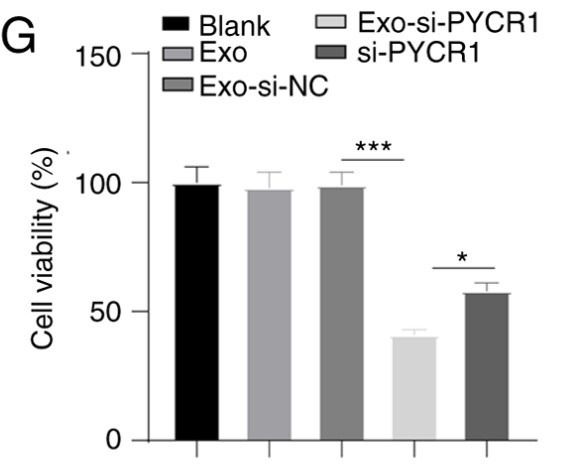
Fig2. MTT assay detected blocked proliferation in Exo-si-PYCR1-treated T24 cells.
RhPYCR1 (Recombinant human pyrroline-5-carboxylate reductase 1) protein has various applications in research and biotechnology due to its function as an enzyme involved in the proline biosynthesis pathway. Here are some potential applications of rhPYCR1 protein:
1. Metabolic engineering: RhPYCR1 protein can be used in metabolic engineering approaches to enhance proline biosynthesis in cells or organisms. This can be useful for the production of proline-rich proteins or for improving stress tolerance in plants or microorganisms.
2. Drug discovery: RhPYCR1 protein can be used as a target for drug discovery efforts, particularly for developing inhibitors or activators of the enzyme. Modulating the activity of PYCR1 could potentially have therapeutic applications in diseases where proline metabolism plays a role.
3. Biomarker development: Changes in PYCR1 expression or activity have been associated with various diseases, including cancer and metabolic disorders. RhPYCR1 protein can be used to study the role of PYCR1 in disease progression and to develop PYCR1-based biomarkers for diagnostic or prognostic purposes.
4. Protein purification and characterization: RhPYCR1 protein can be used for biochemical studies to characterize the enzyme's properties, such as substrate specificity, kinetics, and structure-function relationships. It can also be used for developing purification protocols for isolating endogenous PYCR1 protein from cells or tissues.
5. Assay development: RhPYCR1 protein can be used to develop enzymatic assays for measuring PYCR1 activity in biological samples. These assays can be useful for studying proline metabolism in different physiological or pathological conditions.
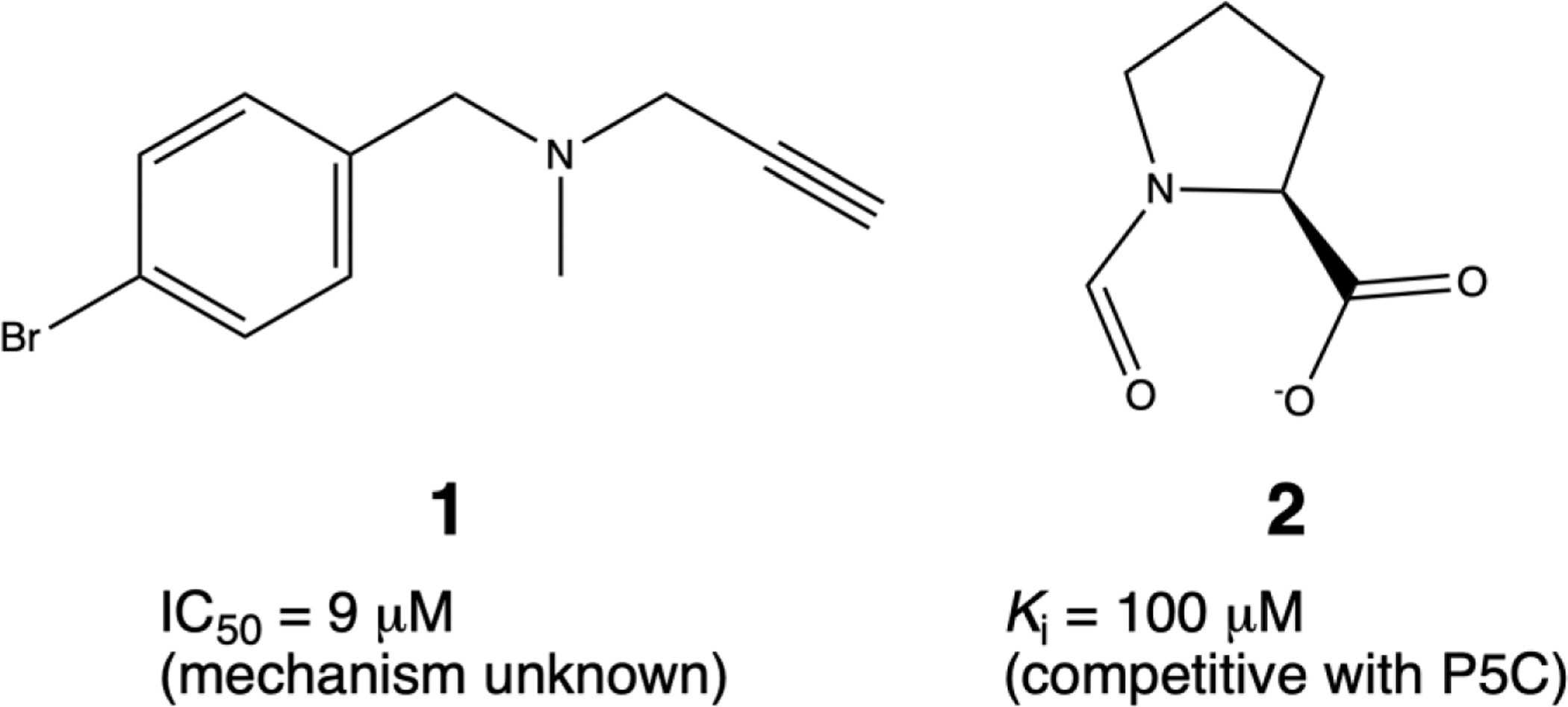
Fig1. Two recently-discovered inhibitors of PYCR1. (Alexandra N Bogner, 2021)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All PYCR1 Products
Required fields are marked with *
My Review for All PYCR1 Products
Required fields are marked with *
Inquiry Basket


