FBN1
-
Official Full Name
fibrillin 1 -
Overview
This gene encodes a member of the fibrillin family. The encoded protein is a large, extracellular matrix glycoprotein that serve as a structural component of 10-12 nm calcium-binding microfibrils. These microfibrils provide force bearing structural support in elastic and nonelastic connective tissue throughout the body. Mutations in this gene are associated with Marfan syndrome, isolated ectopia lentis, autosomal dominant Weill-Marchesani syndrome, MASS syndrome, and Shprintzen-Goldberg craniosynostosis syndrome. -
Synonyms
FBN1;fibrillin 1;FBN, fibrillin 1 (Marfan syndrome) , MFS1, WMS;fibrillin-1;Marfan syndrome;MASS;OCTD;SGS;fibrillin 15;FBN;WMS;MFS1;SSKS;WMS2;ACMICD;GPHYSD2
Recombinant Proteins
- Human
- Mouse
- Rat
- Bovine
- E.coli
- Wheat Germ
- CHO
- HEK293
- NS0
- Mammalian Cells
- His
- GST
- Non
- Fc
- DDK
- Myc
Background
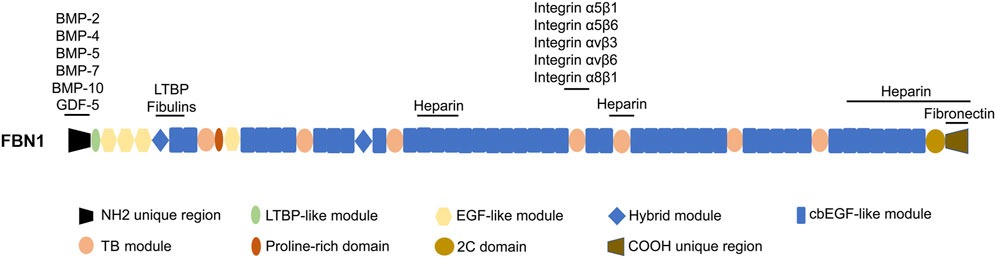
Fig1. The domain structure of FBN1 and the sites responsible for interacting with other proteins. (Li Li, 2024)
What is FBN1 protein?
FBN1 gene (fibrillin 1) is a protein coding gene which situated on the long arm of chromosome 15 at locus 15q21. This gene encodes a member of the fibrillin family of proteins. The encoded preproprotein is proteolytically processed to generate two proteins including the extracellular matrix component fibrillin-1 and the protein hormone asprosin. Fibrillin-1 is an extracellular matrix glycoprotein that serves as a structural component of calcium-binding microfibrils. These microfibrils provide force-bearing structural support in elastic and nonelastic connective tissue throughout the body. Asprosin, secreted by white adipose tissue, has been shown to regulate glucose homeostasis. The FBN1 protein is consisted of 2871 amino acids and FBN1 molecular weight is approximately 312.3 kDa.
What is the function of FBN1 protein?
The FBN1 protein plays a pivotal role in the formation and maintenance of elastic fibers within connective tissues. These elastic fibers provide tissues with their resilience and elasticity, allowing them to stretch and return to their original shape. FBN1 is crucial for the assembly and stabilization of microfibrils, which serve as a scaffold for the deposition of elastin, another key component of elastic fibers. By interacting with various extracellular matrix proteins, growth factors, and proteoglycans, FBN1 helps regulate cell adhesion, migration, and differentiation. It also modulates the bioavailability and activity of transforming growth factor-beta (TGF-β) superfamily members, influencing processes such as fibrosis, angiogenesis, and immune responses. Dysregulation of FBN1 can lead to connective tissue disorders like Marfan syndrome, highlighting its essential role in maintaining tissue architecture and function.
FBN1 related signaling pathway
The FBN1-related signaling pathway is intricately linked to the regulation of extracellular matrix (ECM) homeostasis and tissue remodeling. Fibrillin-1 (FBN1), a major component of microfibrils, interacts with various ECM proteins, growth factors, and proteoglycans, influencing cell adhesion, migration, and differentiation. Through its interaction with transforming growth factor-beta (TGF-β) superfamily members, FBN1 modulates TGF-β bioavailability and activity, impacting downstream signaling pathways involved in fibrosis, angiogenesis, and immune responses. Dysregulation of this pathway, often due to mutations in FBN1, can lead to connective tissue disorders such as Marfan syndrome, characterized by abnormal elastic fiber formation and tissue fragility. Understanding the FBN1-mediated signaling network is crucial for developing therapeutic strategies targeting ECM-related diseases and conditions associated with impaired tissue architecture and function.
FBN1 related diseases
FBN1 is a crucial protein involved in the formation and maintenance of elastic fibers within connective tissues. Mutations or abnormalities in the FBN1 gene are associated with a spectrum of diseases, most notably Marfan syndrome, a genetic disorder characterized by abnormalities in connective tissue that affects the heart, eyes, lungs, and skeletal system. Other conditions linked to FBN1 include Loeys-Dietz syndrome, which shares similarities with Marfan syndrome but involves additional features such as craniofacial abnormalities, and congenital contractural arachnodactyly, another connective tissue disorder. These diseases underscore the importance of FBN1 in maintaining the structural integrity and elasticity of tissues throughout the body.
Bioapplications of FBN1
FBN1 protein, or fibrillin-1, is essential for the integrity of connective tissues as it forms a crucial component of the extracellular matrix. It supports tissue elasticity and plays a key role in regulating the activity of TGF-β, a protein involved in cell growth and differentiation. FBN1 is vital for elastic fiber formation and is implicated in conditions such as Marfan syndrome when defective.
Case Study
Case Study 1: Wenfeng Lin, 2023
Marfan syndrome (MFS), caused by FBN1 mutations, is a connective tissue disorder with unclear molecular mechanisms. This study investigated the role of L-type calcium channels (CaV1.2) in MFS progression and its potential as a treatment target. Here CaV1.2 expression and vascular smooth muscle cell (VSMC) proliferation were reduced with FBN1 deficiency, and this was linked to increased TGF-β1 levels in MFS patients. Modulating Cav1.2 with siRNA and the agonist Bay K8644 showed its role in cell proliferation via c-Fos activity. The results indicate that Cav1.2 could be a therapeutic target for MFS.

Fig1. Representative images of immunofluorescent staining for FBN1 and Cav1.2 in control-HASMCs and MFS-HASMCs.
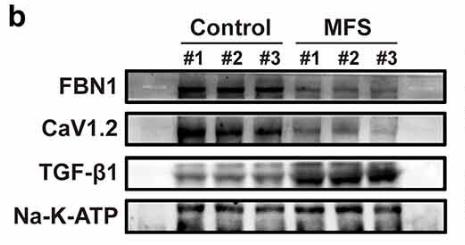
Fig2. Western blot and quantitative analysis of FBN1, Cav1.2 and TGF-β1 protein levels.
Case Study 2: Florian Alonso, 2023
Fibrillin-1, an extracellular matrix protein, is vital for angiogenesis and associated with Marfan syndrome when mutated. This study shows it colocalizes with MAGP1 at the angiogenic front in mouse retinas. In a Marfan model (Fbn1C1041G/+ mice), MAGP1 deposition and endothelial sprouting were reduced, affecting cell phenotypes. Cell culture confirmed fibrillin-1 deficiency disrupts VEGF-A/Notch and Smad signaling, crucial for endothelial cell differentiation. Supplementing with a recombinant fibrillin-1 fragment rescued these defects, affecting proteins like ADAMTS1. The findings highlight the role of fibrillin-1 and MAGP1 in angiogenesis and suggest potential therapeutic approaches for Marfan syndrome.

Fig3. IB4 (green)- and FBN1 (red)-stained angiogenic front at P6.

Fig4. P-Smad1/5 and P-Smad2/3 in FBN1-silenced HMVECs, in the basal state and after 30 min TGF-β or BMP9 stimulation.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (FBN1-3878H)
.
.jpg)
Fig2. SDS-PAGE (FBN1-2622H)
Involved Pathway
FBN1 involved in several pathways and played different roles in them. We selected most pathways FBN1 participated on our site, such as Elastic fibre formation,Extracellular matrix organization,Integrin cell surface interactions, which may be useful for your reference. Also, other proteins which involved in the same pathway with FBN1 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Elastic fibre formation | LTBP3,MFAP2,LOXL4,EFEMP1,MFAP4,ABHD1,LOXL1,BMP10,FURINA,LTBP2 |
| Integrin cell surface interactions | COL23A1,BSG,LOC100514666,COL9A1B,ICAM4,JAM2B,COL13A1,COMP,FN1B,COL16A1 |
| Extracellular matrix organization | FN1B,MFAP4,TLL2,ADAM15,NID2,MGC174152,MUSK,DMD,PCOLCE,NID1 |
Protein Function
FBN1 has several biochemical functions, for example, calcium ion binding,extracellular matrix constituent conferring elasticity,extracellular matrix structural constituent. Some of the functions are cooperated with other proteins, some of the functions could acted by FBN1 itself. We selected most functions FBN1 had, and list some proteins which have the same functions with FBN1. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| protein complex binding | SLC25A3,CASP8,SKIL,SHANK1,RELA,IGBP1,ANKRD32,PDZK1,Cel,RIPK1 |
| protein binding | HDDC2,ETV5,USP14,C16orf53,C10orf46,SERPINE2,RNF168,LMAN1,NR1D2,SYT1 |
| extracellular matrix structural constituent | ELNA,EFEMP2,FBN2B,COL27A1B,MATN1,LAMC1,COL1A2,LAMB1,MEPE,COMP |
| calcium ion binding | ANXA5,F7,EFCAB10,TBC1D9B,MCFD2,CBL,CALRL2,FSTL1A,CABS1,RCN2 |
| extracellular matrix constituent conferring elasticity | FBN2 |
| integrin binding | CTGFB,CYR61L1,ACTN4,MFGE8,MMP14,ADAMTS8,TSPAN4,COL4A3,THBS1,DMP1 |
Interacting Protein
FBN1 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with FBN1 here. Most of them are supplied by our site. Hope this information will be useful for your research of FBN1.
EFEMP2;LOX;ELN;MFAP4;FN1;FBN2;LTBP1;LTBP4;FBLN5
Resources
Related Services
Related Products
References
- Perez-Rico, C; Pascual, G; et al. Elastin Development-Associated Extracellular Matrix Constituents of Subepithelial Connective Tissue in Human Pterygium. INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE 55:6309-6318(2014).
- Beene, LC; Wang, LW; et al. Nonselective Assembly of Fibrillin 1 and Fibrillin 2 in the Rodent Ocular Zonule and in Cultured Cells: Implications for Marfan Syndrome. INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE 54:8337-8344(2013).



