Thymus-Dependent B Cell Activation
Related Symbol Search List
- EMR2
- CD97
- BTLA
- CD226
- CD276
- CD28
- CD37
- CD40
- CD48
- CD72
- CD80
- CD84
- CD86
- Ctla4
- HLA-DRA
- Icosl
- LY9
- MS4A1
- PDCD1
- SPN
- Tnfsf4
- TNFSF9
Immunology Background
Background
Thymus-dependent B cell activation refers to the process by which B cells require interaction with T cells, specifically CD4+ helper T cells, for their full activation and differentiation. This process is significant for the generation of high-affinity antibodies, memory B cells, and long-term immune protection.
Process of Thymus-Dependent B Cell Activation
Here's an overview of the process and its significance:
| Process | Details |
|---|---|
| 1. Antigen Recognition: | Thymus-dependent B cell activation begins with the recognition of an antigen by the B cell receptor (BCR). The BCR is composed of membrane-bound immunoglobulin (Ig) molecules that bind to specific antigens. The BCR recognizes and captures antigens, internalizing them into the B cell. |
| 2. Antigen Presentation | Once the antigen is internalized, the B cell processes it into smaller peptide fragments. These peptides are then presented on the B cell surface using major histocompatibility complex class II (MHC-II) molecules. The antigen-MHC-II complex acts as a signal to attract and activate CD4+ helper T cells. |
| 3. T-B Cell Interaction | CD4+ helper T cells recognize the antigen-MHC-II complex on the B cell surface through their T cell receptor (TCR). This interaction is further strengthened by co-stimulatory molecules on both cells, such as CD40-CD40L and CD28-B7 interactions. |
| 4. T Cell Help | The interaction between the B cell and CD4+ helper T cell provides critical help signals to the B cell. The CD40-CD40L interaction triggers signaling pathways that enhance B cell activation, proliferation, and antibody class switching. Cytokines secreted by CD4+ helper T cells, such as interleukin-4 (IL-4) and interleukin-21 (IL-21), further support B cell differentiation and antibody production. |
| 5. Germinal Center Formation | Thymus-dependent B cell activation leads to the formation of germinal centers within secondary lymphoid organs, such as lymph nodes and spleen. Germinal centers are specialized microenvironments where B cells undergo intense proliferation, affinity maturation, and class switching. |
| 6. Somatic Hypermutation and Affinity Maturation | Within germinal centers, B cells undergo somatic hypermutation, a process where the variable regions of the BCR genes undergo random mutations. B cells with BCRs that have higher affinity for the antigen are positively selected and receive survival signals, while those with lower affinity undergo apoptosis. This process leads to the generation of B cells with increasingly higher affinity for the antigen. |
| 7. Differentiation and Antibody Production | Germinal center B cells can further differentiate into memory B cells or plasma cells. Memory B cells provide long-term immune memory, enabling a rapid and robust response upon re-exposure to the antigen. Plasma cells, on the other hand, are terminally differentiated B cells that produce and secrete large amounts of antibodies specific to the antigen. |
The significance of thymus-dependent B cell activation lies in its ability to generate high-affinity antibodies with diverse specificities, as well as memory B cells that confer long-term immunity. This process ensures an effective immune response against pathogens and provides the basis for vaccination strategies. Thymus-dependent B cell activation also allows for the regulation and fine-tuning of immune responses through the interaction between B cells and helper T cells. Furthermore, defects in thymus-dependent B cell activation can lead to immunodeficiency disorders, impairing the body's ability to mount effective immune responses.
Differences Between Thymus-Dependent and Thymus-Independent B Cell Activation Pathways
Thymus-dependent (TD) and thymus-independent (TI) B cell activation pathways differ in several key aspects, including the requirement for T cell help, the nature of the antigens involved, the type of immune response generated, and the affinity maturation process. Here are the main differences between the two pathways:
| TD B cell activation pathways | TI B cell activation pathways | |
|---|---|---|
| Mechanism | TD B cell activation requires T cell help for optimal activation and differentiation. It involves specific interactions between B cells and CD4+ helper T cells. | TI B cell activation occurs without the requirement of T cell help. It can be further divided into two subtypes:
|
| T Cell Dependence | TD B cell activation relies on the interaction between B cells and CD4+ helper T cells. B cells present antigenic peptides to CD4+ T cells via major histocompatibility complex class II (MHC-II) molecules. This interaction triggers T cell activation and subsequent help for B cell activation. | TI B cell activation occurs in the absence of T cell help, hence the name "thymus-independent." It does not require the interaction between B cells and CD4+ helper T cells. |
| Antibody Response | TD B cell activation leads to the production of different antibody isotypes, including IgG, IgA, and IgE, through class switching. It also allows for affinity maturation, which improves the antibody's binding affinity to the antigen. | TI B cell activation leads to the production of primarily IgM antibodies. Class switching (changing the antibody isotype) and affinity maturation (improving antibody affinity) are limited in TI responses. |
| T Cell Help | TD B cell activation requires interaction with CD4+ helper T cells for full activation and differentiation. | In contrast, TI B cell activation occurs in the absence of T cell help. |
| Antigens | TD B cell activation primarily occurs in response to protein antigens. Protein antigens require processing and presentation on major histocompatibility complex class II (MHC-II) molecules to activate CD4+ helper T cells. | TI B cell activation can occur in response to certain types of antigens, including polysaccharides, lipopolysaccharides (LPS), and repetitive epitopes. These antigens can directly stimulate B cells without the need for antigen processing or T cell help. |
| Immune Response | TD B cell activation leads to the generation of high-affinity antibodies and the formation of memory B cells. The immune response generated is typically characterized by class-switched antibodies, affinity maturation, and long-term immune memory. | TI B cell activation generally results in the production of lower-affinity antibodies and a limited or absent memory B cell response. The immune response is often dominated by immunoglobulin M (IgM) antibodies and lacks affinity maturation. |
| Affinity Maturation | TD B cell activation involves the process of affinity maturation, which occurs within germinal centers. Somatic hypermutation and selection of B cells with higher-affinity B cell receptors (BCRs) for the antigen lead to the generation of B cells with improved binding affinity. | TI B cell activation does not involve significant affinity maturation, as the process of somatic hypermutation and germinal center formation is limited or absent. |
| Co-Stimulatory Signals | TD B cell activation requires co-stimulatory signals, such as CD40-CD40L interaction and cytokines from CD4+ helper T cells, to fully activate B cells. | Co-stimulatory signals are not as critical in TI B cell activation. |
| Memory B Cell Generation | TD B cell activation generates memory B cells, which provide long-term immune memory and enable a rapid secondary immune response upon re-exposure to the antigen. | TI B cell activation often does not generate robust memory B cell responses, resulting in a weaker secondary immune response. |
In summary, the main differences between thymus-dependent and thymus-independent B cell activation pathways lie in the requirement for T cell help, the nature of antigens, the type of immune response generated, the process of affinity maturation, and the generation of memory B cells. Thymus-dependent B cell activation is more complex and results in a more diverse and potent immune response, while thymus-independent B cell activation is simpler and typically leads to a less specialized immune response.
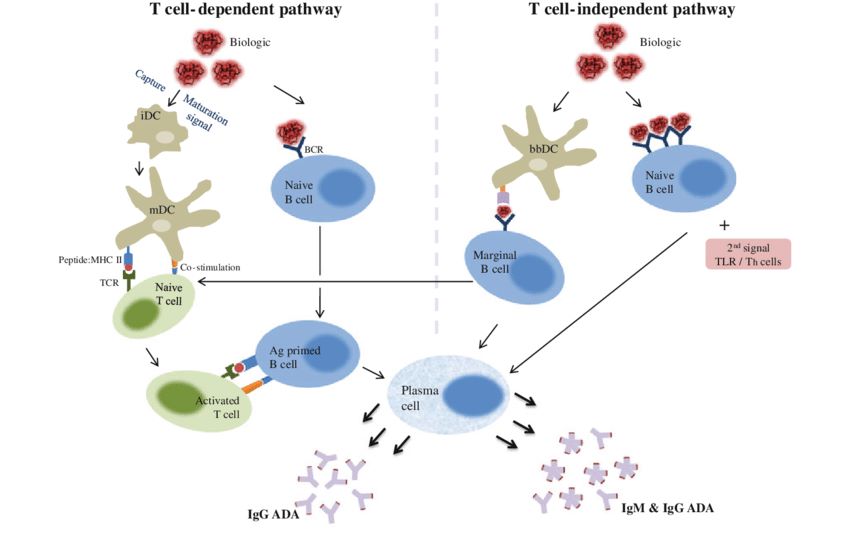 Fig.1 T cell-dependent and independent pathway involved in an immunogenic response. (Sethu S, et al., 2012)
Fig.1 T cell-dependent and independent pathway involved in an immunogenic response. (Sethu S, et al., 2012)Molecules Associated with Thymus-Dependent B Cell Activation
There are several types of molecules associated with thymus-dependent (TD) B cell activation, each with specific functions. Here are the main types and their functions:
| Types | Functions |
|---|---|
| Co-stimulatory Molecules |
|
| Inhibitory Receptors |
|
| Antigen Presentation Molecules |
|
| Receptors for Cell-Cell Interactions |
|
| Cytokines and Cytokine Receptors |
|
| B Cell Receptor (BCR) and Associated Molecules |
|
These different types of molecules work together to orchestrate thymus-dependent B cell activation. Co-stimulatory molecules provide positive signals, inhibitory receptors regulate immune responses, antigen presentation molecules facilitate T cell collaboration, receptors for cell-cell interactions mediate communication, cytokines drive B cell differentiation, and the BCR and associated molecules directly engage with antigens. This coordinated network ensures effective B cell activation and the generation of specific immune responses.
The Significance of Thymus-Dependent B-cell Activation and Related Molecules in Disease
Thymus-dependent (TD) B-cell activation and related molecules play significant roles in various diseases. Here are some key aspects of their significance in disease:
Infectious Diseases
TD B-cell activation is vital for mounting effective immune responses against infectious diseases. The interaction between B cells and CD4+ helper T cells, facilitated by molecules such as CD40 and CD40L, is crucial for generating high-affinity antibodies and establishing immunological memory. Defects in TD B-cell activation or related molecules can lead to impaired antibody production and susceptibility to recurrent or severe infections.
Autoimmune Disorders
Dysregulation of TD B-cell activation and associated molecules can contribute to the development of autoimmune disorders. Abnormal activation of autoreactive B cells and the production of autoantibodies are key features of autoimmune diseases. Molecules involved in TD B-cell activation, such as CD40/CD40L, BAFF, IL-21, and TLRs, play roles in promoting autoreactive B-cell responses and the breakdown of self-tolerance. Targeting these molecules or their signaling pathways is a potential therapeutic approach for managing autoimmune diseases.
Allergic Diseases
TD B-cell activation is implicated in the pathogenesis of allergic diseases. In allergic responses, B cells produce allergen-specific antibodies, including IgE, which trigger mast cell and basophil activation, leading to the release of inflammatory mediators. Molecules like CD40/CD40L, BAFF, and cytokines (e.g., IL-4, IL-6) are involved in the activation and differentiation of allergen-specific B cells. Modulating these molecules could offer therapeutic strategies for mitigating allergic diseases.
Cell Malignancies
Dysregulated TD B-cell activation and related molecules can contribute to the development of B-cell malignancies, including B-cell lymphomas and leukemias. Aberrant signaling through CD40, BAFF, or IL-21 receptors can promote uncontrolled B-cell proliferation and survival. Additionally, genetic alterations affecting molecules involved in TD B-cell activation can lead to the malignant transformation of B cells. Targeting these molecules or their downstream signaling pathways is a potential approach for treating B-cell malignancies.
Vaccination
TD B-cell activation is critical for the success of vaccination. Vaccines often aim to induce strong and long-lasting antibody responses, which rely on TD B-cell activation and the generation of memory B cells. Understanding the molecules involved in TD B-cell activation can help in the design of effective vaccines and adjuvants that enhance B-cell responses.
Therapeutic Interventions
Targeting molecules involved in TD B-cell activation offers opportunities for therapeutic interventions. Modulating the activity or expression of these molecules can help regulate B-cell responses in autoimmune diseases, allergic diseases, and B-cell malignancies. Additionally, stimulating TD B-cell activation pathways can be explored to enhance vaccine responses or develop immunotherapies against infectious diseases or cancer.
In summary, the significance of TD B-cell activation and related molecules in disease lies in their roles in immune responses, autoimmunity, allergic diseases, B-cell malignancies, vaccination, and potential therapeutic interventions. Understanding these processes can aid in the development of novel treatment strategies and therapeutic interventions for a range of diseases.
Case Study
Case 1: Marasco E, Farroni C, Cascioli S, et al. B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients. Eur J Immunol. 2017;47(1):131-143.
In order to study the functions of the B-cell compartment in vitro, the authors chose to activate B cells with either TI and/or TD signals. CpG ODN-2006, a synthetic oligonucleotide that binds to TLR9, was chosen as a representative TI stimulus; whereas the TD signal was mimicked by engaging CD40 on B cells. When B cells were activated with both CpG and CD40L, CD40L inhibited CpG-induced plasmablast differentiation, immunoglobulins secretion, and the expression of PRDM1 (A–E). On the other hand, in the presence of CpG, CD40L-induced AID upregulation did not occur (E).
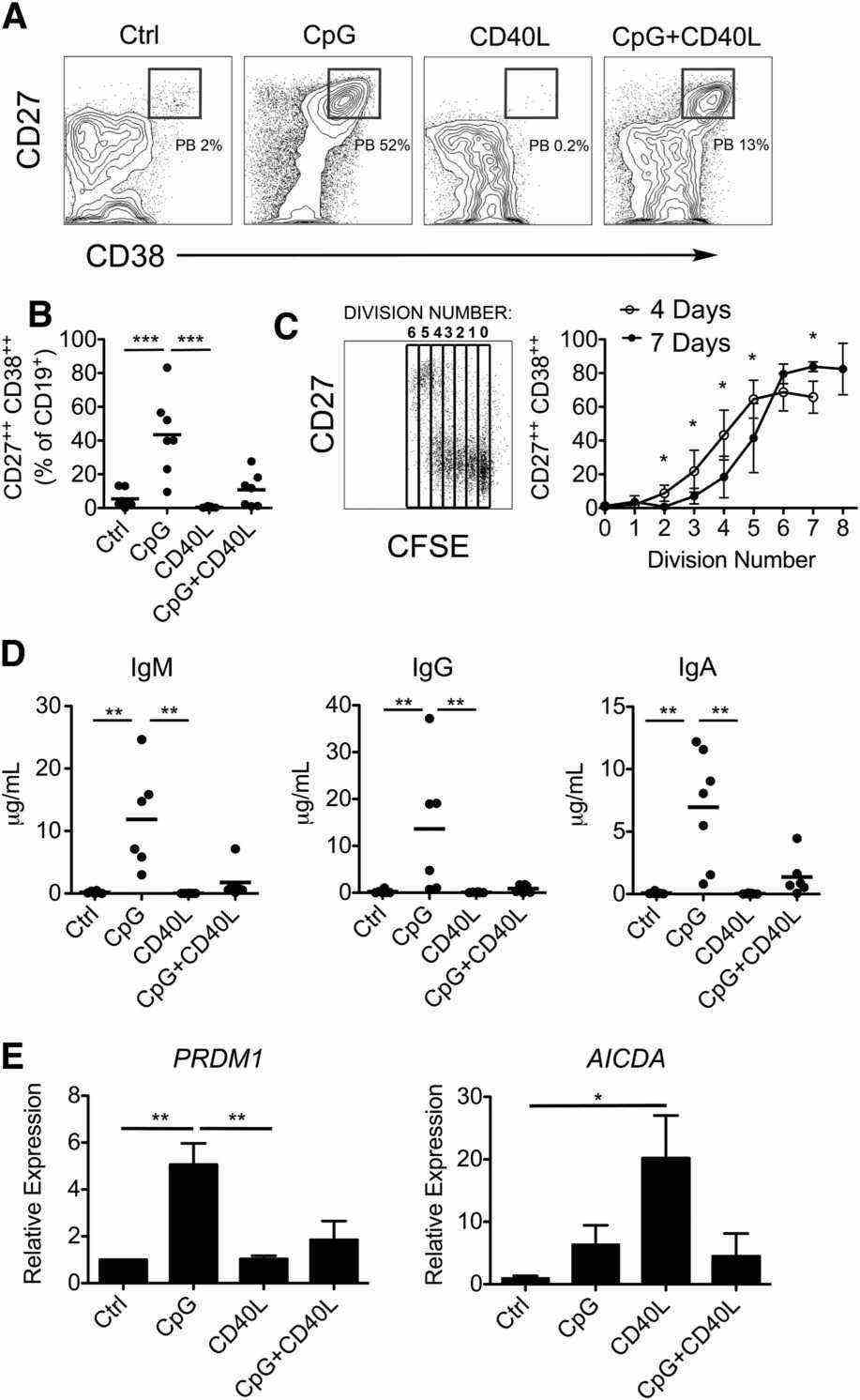 Fig.2 In vitro activation of B cells with TI and TD stimulation.
Fig.2 In vitro activation of B cells with TI and TD stimulation.Case 2: Marsman C, Verhoeven D, Koers J, et al. Optimized protocols for in-vitro T-cell-dependent and T-cell-independent activation for B-cell differentiation studies using limited cells. Front Immunol. 2022;13:815449.
In an attempt to drive differentiation and expansion even further in our one-step in-vitro B-cell differentiation assay, the effect of additional stimuli in our culture conditions was tested. For this purpose, the reference stimuli CD40L and IL-21 were combined with or without F(ab)2 fragments targeting IgM, IgG, and IgA to induce BCR signaling (also referred to as anti-BCR). Secondly, we tested whether the addition of IL-4, a cytokine important for naive B cells during the GC reactions, can augment in-vitro B-cell differentiation induced by CD40L and IL-21.
In conclusion, an augmenting effect of anti-BCR on TD-induced B-cell differentiation was found, but its use prevented Ig secretion analysis. Notably, although we observed no significant effect on plasmablast differentiation, IL-4 reduced Ig secretion under all conditions tested.
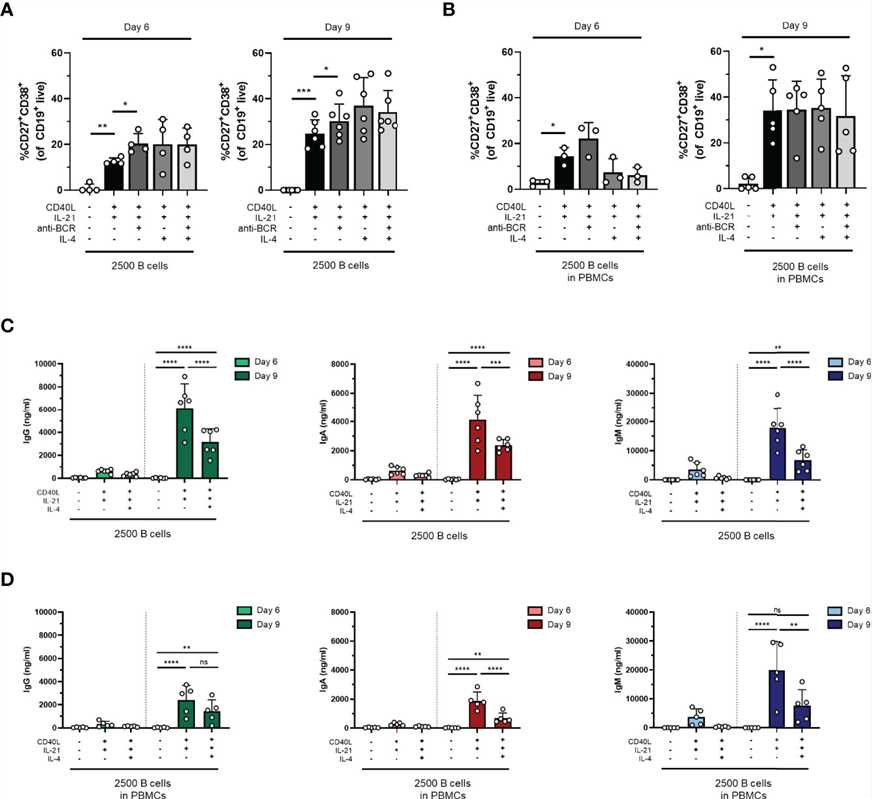 Fig.3 The addition of anti-BCR stimuli in a T-cell-dependent stimulation results in increased B-cell differentiation, while IL-4 severely inhibits antibody production.
Fig.3 The addition of anti-BCR stimuli in a T-cell-dependent stimulation results in increased B-cell differentiation, while IL-4 severely inhibits antibody production.References
- Sethu S, Govindappa K, Alhaidari M, Pirmohamed M, Park K, Sathish J. Immunogenicity to biologics: mechanisms, prediction and reduction. Arch Immunol Ther Exp (Warsz). 2012;60(5):331-344.
- Marsman C, Verhoeven D, Koers J, et al. Optimized protocols for in-vitro T-cell-dependent and T-cell-independent activation for B-cell differentiation studies using limited cells. Front Immunol. 2022;13:815449.
