Recombinant Human EFNA1 protein(Met1-Ser182), His-tagged
| Cat.No. : | EFNA1-2238H |
| Product Overview : | Recombinant Human Ephrin-A1 (NP_004419.2)(Met 1-Ser182) was expressed in HEK293, fused with a polyhistidine tag at the C-terminus. |
| Availability | April 21, 2025 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | HEK293 |
| Tag : | His |
| Protein Length : | 1-182 a.a. |
| Form : | Lyophilized from sterile PBS, pH 7.4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Bio-activity : | Measured by its binding ability in a functional ELISA. Immobilized Human Ephrin-A1 His at 2 μg/ml (100 μl/well) can bind Human EphA1 hFc, the EC50 of Human EphA1 hFc is 8.0-48.0 ng/mL. |
| Molecular Mass : | The recombinant human Ephrin-A1 comprises 175 amino acids a predicted molecular mass of 20.8 kDa. As a result of glycosylation, rh Ephrin-A1 migrates as an approximately 26 kDa band in SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 EU per μg of the protein as determined by the LAL method |
| Purity : | > 97 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | EFNA1 ephrin-A1 [ Homo sapiens ] |
| Official Symbol | EFNA1 |
| Synonyms | EFNA1; ephrin-A1; EPLG1, TNFAIP4; ECKLG; LERK1; TNF alpha-induced protein 4; ligand of eph-related kinase 1; immediate early response protein B61; eph-related receptor tyrosine kinase ligand 1; tumor necrosis factor alpha-induced protein 4; tumor necrosis factor, alpha-induced protein 4; B61; EFL1; EPLG1; LERK-1; TNFAIP4; |
| Gene ID | 1942 |
| mRNA Refseq | NM_004428 |
| Protein Refseq | NP_004419 |
| MIM | 191164 |
| UniProt ID | P20827 |
| ◆ Recombinant Proteins | ||
| EFNA1-328H | Active Recombinant Human EFNA1 protein, hFc-tagged | +Inquiry |
| EFNA1-28470TH | Recombinant Human EFNA1, His-tagged | +Inquiry |
| EFNA1-136HF | Recombinant Full Length Human EFNA1 Protein | +Inquiry |
| EFNA1-469R | Recombinant Rat EFNA1 Protein (18-182 aa), GST-tagged | +Inquiry |
| EFNA1-2024R | Recombinant Rat EFNA1 Protein | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| EFNA1-1514RCL | Recombinant Rat EFNA1 cell lysate | +Inquiry |
| EFNA1-2177MCL | Recombinant Mouse EFNA1 cell lysate | +Inquiry |
| EFNA1-2594HCL | Recombinant Human EFNA1 cell lysate | +Inquiry |
Case 1: Tandon M, et al. J Gene Med. 2012
Combined EphrinA1-Fc and Flt3L adenoviral therapy synergistically inhibits breast cancer growth by activating EphA2 degradation and dendritic cell-mediated immunity, demonstrating enhanced anti-tumor immune responses in preclinical models. This dual-targeted approach highlights potential for EphA2-directed immunotherapy and combination strategies in oncology.
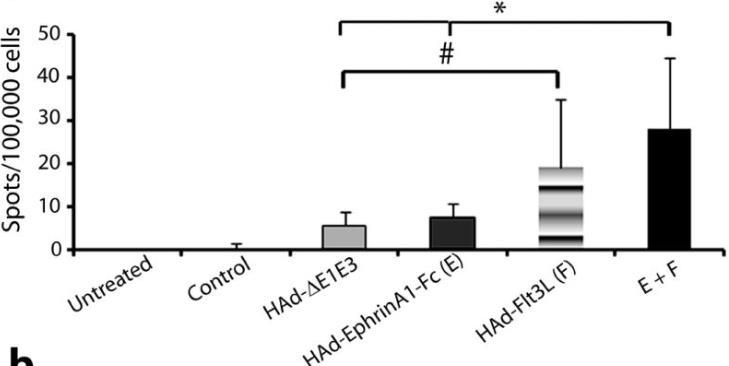
Fig1. The tumor bearing mice were inoculated i.t. three times with PBS, HAd-ΔE1E3, HAd-EphrinA1-Fc, HAd-Flt3L, or HAd-EphrinA1-Fc + HAd-Flt3L.
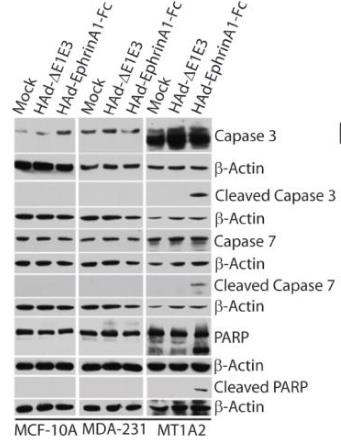
Fig2. MCF-10A, MDA-MB-231 and MT1A2 cells were either mock-infected or infected with 100 pfu/cell of HAd-ΔE1E3 or HAd-EphrinA1-Fc.
Case 2: Wiedemann E, et al. Cell Signal. 2017
Ephrin-A1 regulates endothelial proliferation/migration via EphA2 signaling and cytoskeletal dynamics, suppressing growth while enhancing directed cell movement—key for angiogenesis and vascular wound healing. Its density-dependent expression modulates re-endothelialization, offering therapeutic targets for vascular repair and anti-angiogenic strategies.
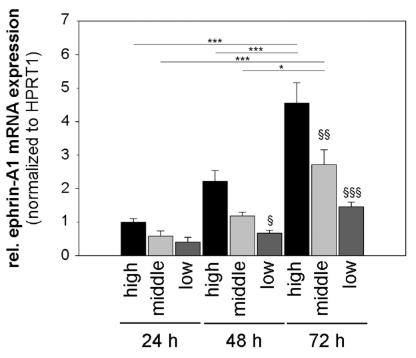
Fig1. The most pronounced effect was seen in the case of ephrin-A1 which correlates highly with the cell density.
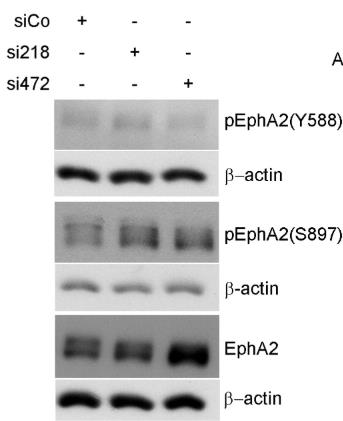
Fig2. Silencing of ephrin-A1 (si218 and si472) leads to an increased EphA2 expression in endothelial cells.
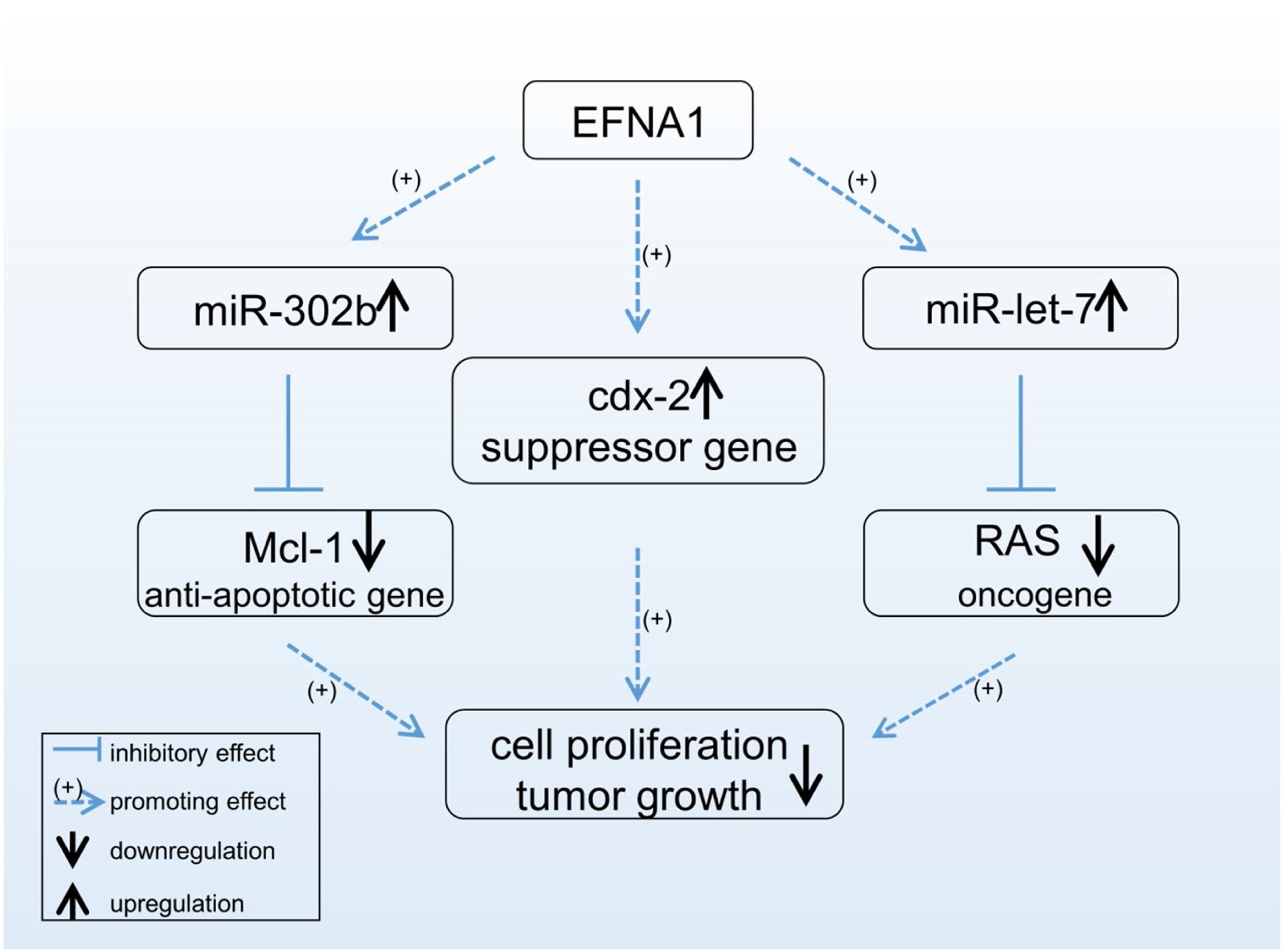
Fig1. Possible mechanisms by which EFNA1 inhibits tumor growth. (Yongping Hao, 2020)
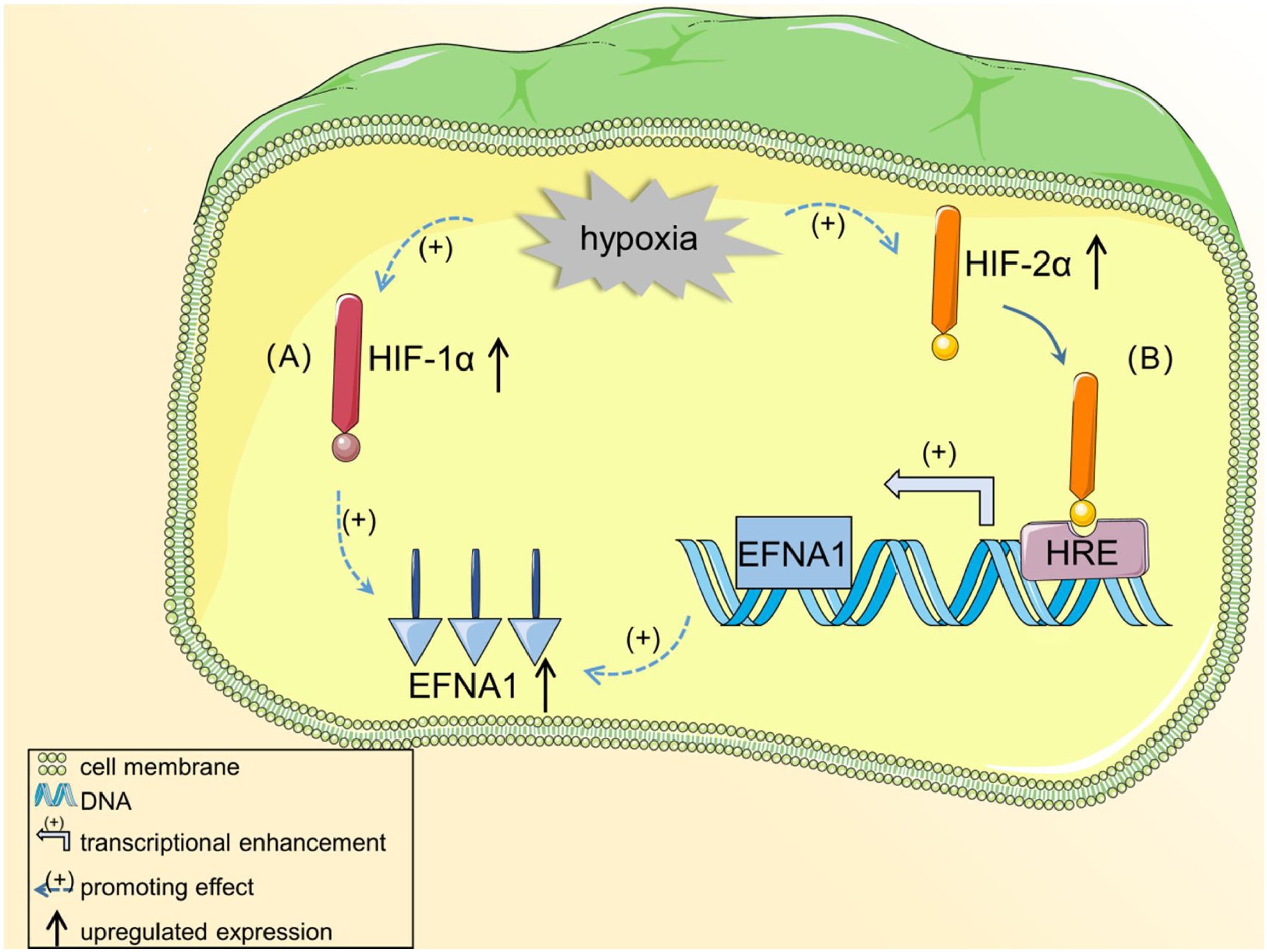
Fig2. EFNA1 is induced in hypoxic environments in HIF-dependent pathways. (Yongping Hao, 2020)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All EFNA1 Products
Required fields are marked with *
My Review for All EFNA1 Products
Required fields are marked with *
Inquiry Basket


