Recombinant Human DARS protein(Met1-Pro501), His-tagged
| Cat.No. : | DARS-564H |
| Product Overview : | Recombinant Human DARS (P14868) (Met1-Pro501) was expressed in E. coli with a polyhistide tag at the N-terminus. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | Met1-Pro501 |
| Form : | Lyophilized from sterile 50mM Tris, 100mM Nacl, 10% glycerol, pH 8.0. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human DARS consists of 516 amino acids and predicts a molecular mass of 59 KDa. It migrates as an approximately 47 KDa band in SDS-PAGE under reducing conditions. |
| Purity : | > 90 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | DARS aspartyl-tRNA synthetase [ Homo sapiens ] |
| Official Symbol | DARS |
| Synonyms | DARS; aspartyl-tRNA synthetase; aspartate--tRNA ligase, cytoplasmic; aspartate tRNA ligase 1; cytoplasmic; aspRS; aspartate tRNA ligase 1, cytoplasmic; aspartyl-tRNA synthetase, cytoplasmic; cell proliferation-inducing protein 40; cell proliferation-inducing gene 40 protein; MGC111579; DKFZp781B11202; |
| Gene ID | 1615 |
| mRNA Refseq | NM_001349 |
| Protein Refseq | NP_001340 |
| MIM | 603084 |
| UniProt ID | P14868 |
| ◆ Recombinant Proteins | ||
| DARS-1920H | Recombinant Human DARS Protein (Gly363-Pro501), His tagged | +Inquiry |
| DARS-11831H | Recombinant Human DARS, GST-tagged | +Inquiry |
| DARS-4805Z | Recombinant Zebrafish DARS | +Inquiry |
| DARS-2514HF | Recombinant Full Length Human DARS Protein, GST-tagged | +Inquiry |
| DARS-1666C | Recombinant Chicken DARS | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| DARS-7073HCL | Recombinant Human DARS 293 Cell Lysate | +Inquiry |
Case 1: Guzzo CM, et al. Biochem Biophys Res Commun. 2008
Human lysyl-tRNA synthetase (hKRS) requires N-terminal truncation (hKRSΔ60) to bind EF1α and enhance lysylation activity, while interactions with aspartyl-tRNA synthetase (AspRS) and p38 from the multi-enzyme complex reveal distinct regulatory effects on catalysis. This study highlights the structural evolution of multi-enzyme complexes, emphasizing appended extensions’ roles in functional modulation and tRNA synthetase network dynamics, with implications for understanding enzyme assembly mechanisms and molecular interactions in translational regulation.
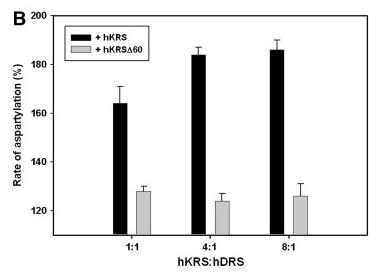
Fig1. Stimulation of AspRS activity by hKRS.
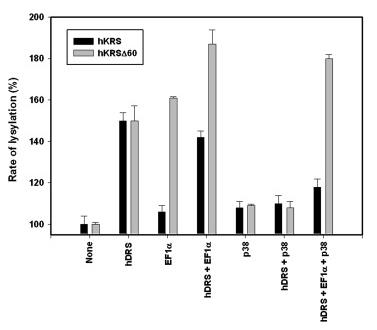
Fig2. Combined effects of AspRS, EF1α, and p38 on lysylation by hKRS.
Case 2: Liu B, et al. J Pathol. 2022
Despite limited targeted therapies for gastric cancer (GC), NGS-driven functional genomics screening with a metabolism-focused shRNA library identified aspartyl-tRNA synthetase (DARS) as a novel therapeutic target. High DARS expression correlates with aggressive tumor progression and poor prognosis in diffuse-type GC patients. In vitro and in vivo studies confirmed that DARS suppression inhibits cancer growth via p-ERK pathway inactivation, extending its potential applicability to other malignancies. This highlights DARS as both a prognostic biomarker and a promising therapeutic target for precision oncology strategies.
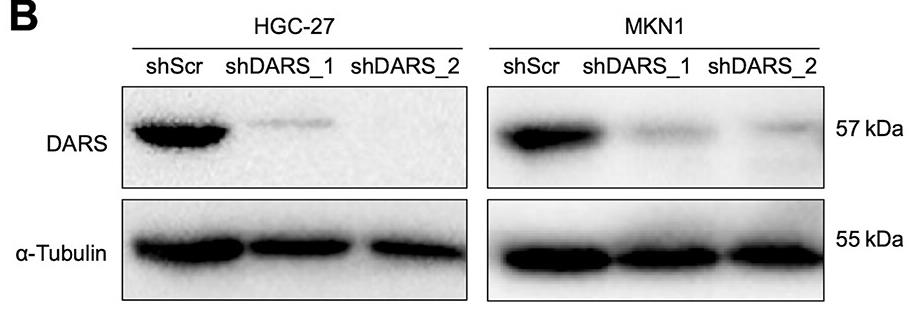
Fig1. Immunoblots for DARS and α-tubulin are shown with their molecular sizes.
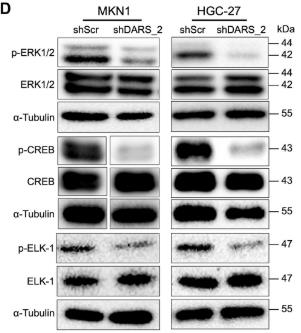
Fig2. Immunoblots of CREB, ERK1/2, and ELK-1 proteins with molecular sizes (kDa) are shown for MKN1 and HGC-27 cells infected by either shScr or shDARS_2.
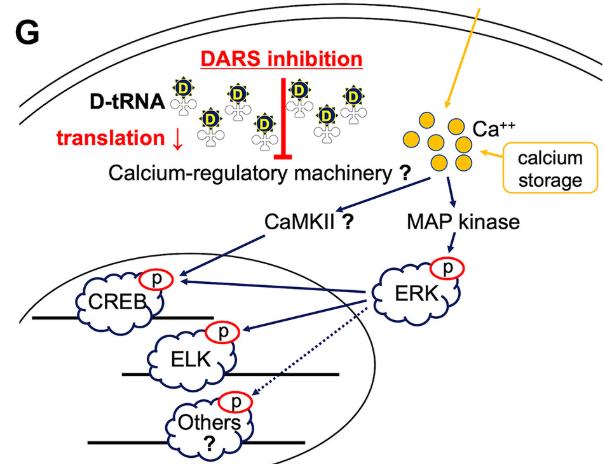
Fig1. DARS inhibition downregulates multiple MAPK downstream, hypothetically via Ca2+ regulatory pathways. (Bin Liu, 2022)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All DARS Products
Required fields are marked with *
My Review for All DARS Products
Required fields are marked with *
Inquiry Basket


