Recombinant Human CST7 protein(Met1-His145), hFc-tagged
| Cat.No. : | CST7-679H |
| Product Overview : | Recombinant Human CST7 (NP_003641.3) (Met 1-His 145) was expressed in HEK293 with the fused Fc region of Human IgG1 at the C-terminus. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | HEK293 |
| Tag : | Fc |
| Protein Length : | 1-145 a.a. |
| Form : | Lyophilized from sterile PBS, pH7. 4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Bio-activity : | Measured by its ability to inhibit active Cathepsin L cleavage of a fluorogenic peptide substrate Z-LR-AMC. The IC50 value is <5 nM. |
| Molecular Mass : | The recombinant human Cystatin-F/Fc is a disulfide-linked homodimeric protein. The reduced monomer consists of 364 amino acids and predicts a molecular mass of 41.3 kDa. As a result of glycosylation, the rh Cystatin-F/Fc monomer migrates as an approximately 50 kDa band in SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 EU per μg of the protein as determined by the LAL method |
| Purity : | > 97 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | CST7 cystatin F (leukocystatin) [ Homo sapiens ] |
| Official Symbol | CST7 |
| Synonyms | CST7; cystatin F (leukocystatin); cystatin-F; cystatin-7; leukocystatin; cystatin-like metastasis-associated protein; CMAP; |
| Gene ID | 8530 |
| mRNA Refseq | NM_003650 |
| Protein Refseq | NP_003641 |
| MIM | 603253 |
| UniProt ID | O76096 |
| ◆ Recombinant Proteins | ||
| CST7-2250H | Recombinant Human CST7 Protein (Gly20-His145), C-His tagged | +Inquiry |
| CST7-3150H | Recombinant Human CST7 protein, His-tagged | +Inquiry |
| CST7-3149H | Recombinant Human CST7, His tagged | +Inquiry |
| CST7-2240HF | Recombinant Full Length Human CST7 Protein, GST-tagged | +Inquiry |
| Cst7-2035M | Recombinant Mouse Cst7 Protein, His (Fc)-Avi-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| CST7-1522MCL | Recombinant Mouse CST7 cell lysate | +Inquiry |
| CST7-2472HCL | Recombinant Human CST7 cell lysate | +Inquiry |
Case 1: Senjor E, et al. Cell Mol Life Sci. 2023
Cystatin F, a cysteine protease inhibitor, regulates NK cell cytotoxicity via N-glycosylation. High-mannose glycosylation in NK-92 cells drives lysosomal retention, suppressing cathepsin C and cytotoxicity, while complex glycosylation in super-charged NKs promotes secretory pathways, preserving tumor-killing potential. Kifunensine-induced high-mannose glycans reduced cytotoxicity (p<0.01), whereas mannose-6-phosphate blocked cystatin F uptake. Glycosylation modulation enhances NK cell therapy efficacy for cancer immunotherapy.
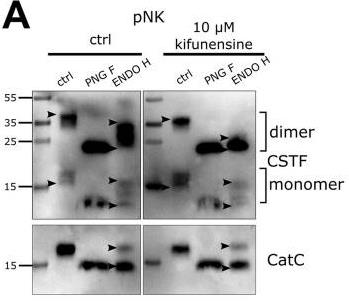
Fig1. Western blot of cystatin F (CSTF) and cathepsin C (CatC) in cell lysates of primary NK (pNK) cells.
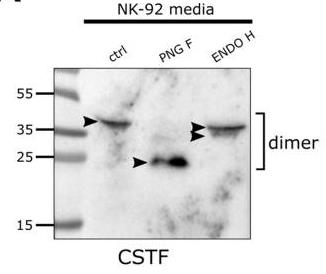
Fig2. Western blot of cystatin F (CSTF) expression in cell media secreted by NK-92 cells.
Case 2: Prunk M, et al. Radiol Oncol. 2019
Cystatin F, a cysteine protease inhibitor in cytotoxic lymphocytes, suppresses cathepsins C/H/L to block granzyme B activation and perforin function in cancer cell killing. In TALL-104 cells, reduced cytotoxicity (via ionomycin/TGF-β treatment) correlated with elevated cystatin F levels and attenuated cathepsin/granzyme B activities. Immunoprecipitation and confocal microscopy confirmed cystatin F-cathepsin interactions, highlighting its role in modulating tumor immunity. Targeting cystatin F-glycosylation pathways may enhance adoptive cell therapy efficacy.
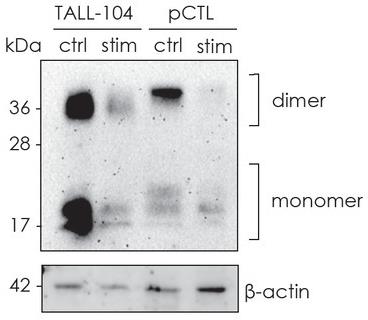
Fig1. Representative western blot experiment showing expression of the monomeric and dimeric form of cystatin F.
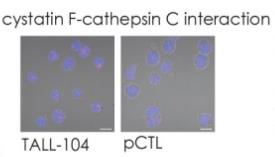
Fig2. Proximity ligation experiment for cystatin F-cathepsin C interaction in TALL-104 cells and pCTLs.
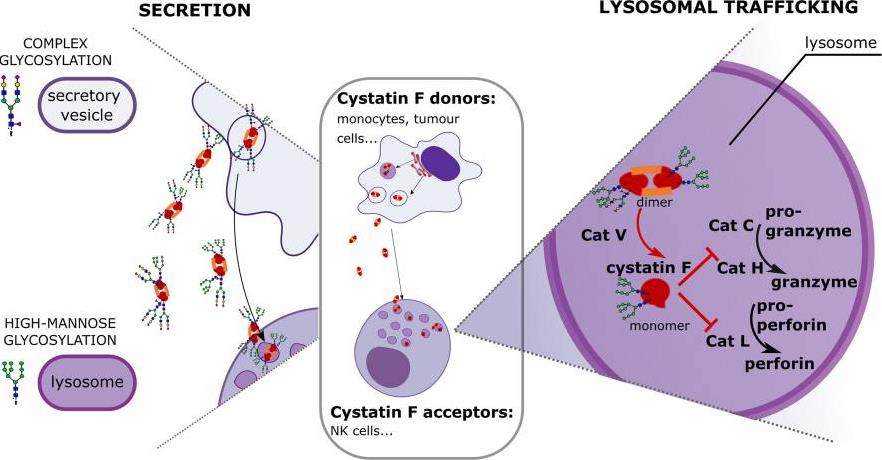
Fig1. Different glycosylation profiles of cystatin F alter the cytotoxic potential of natural killer cells. (Emanuela Senjor, 2023)
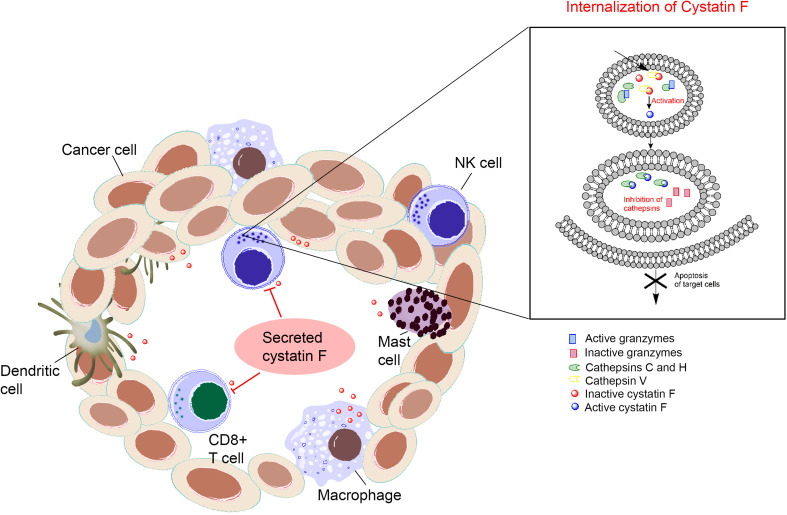
Fig2. Increasing extracellular concentration of cystatin F negatively affects the granule-mediated cytotoxicity of NK cells and CTLs. (Janko Kos, 2018)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All CST7 Products
Required fields are marked with *
My Review for All CST7 Products
Required fields are marked with *
Inquiry Basket


