Recombinant Human CNPY4 protein(Met1-Leu248), His-tagged
| Cat.No. : | CNPY4-8579H |
| Product Overview : | Recombinant Human CNPY4 (Q8N129) (Met1-Leu248) was expressed in HEK293 with a polyhistidine tag at the C-terminus. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | HEK293 |
| Tag : | His |
| Protein Length : | Met1-Leu248 |
| Form : | Lyophilized from sterile PBS, pH 7.4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human CNPY4 consists of 238 amino acids and predicts a molecular mass of 27.4 KDa. It migrates as an approximately 32 KDa band in SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 EU per μg of the protein as determined by the LAL method. |
| Purity : | > 85 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | CNPY4 canopy 4 homolog (zebrafish) [ Homo sapiens ] |
| Official Symbol | CNPY4 |
| Synonyms | PRAT4B |
| Gene ID | 245812 |
| mRNA Refseq | NM_152755.1 |
| Protein Refseq | NP_689968.1 |
| MIM | 610047 |
| UniProt ID | Q8N129 |
| ◆ Recombinant Proteins | ||
| CNPY4-1595H | Recombinant Human CNPY4 Protein, GST-tagged | +Inquiry |
| CNPY4-1957HF | Recombinant Full Length Human CNPY4 Protein, GST-tagged | +Inquiry |
| CNPY4-3620Z | Recombinant Zebrafish CNPY4 | +Inquiry |
| CNPY4-1822M | Recombinant Mouse CNPY4 Protein, His (Fc)-Avi-tagged | +Inquiry |
| CNPY4-771R | Recombinant Rhesus Macaque CNPY4 Protein, His (Fc)-Avi-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| CNPY4-1284HCL | Recombinant Human CNPY4 cell lysate | +Inquiry |
Case 1: Lo M, et al. Nat Commun. 2022
The Hedgehog (HH) pathway is crucial for development and maintaining tissues. Problems with this pathway can lead to defects or cancer. Cholesterol is key for HH activity, but its control is unclear. Researchers discovered Canopy4 (CNPY4) affects HH activity by managing membrane lipids. Without Cnpy4, embryos have defects like changes in digit number. Reducing Cnpy4 ramps up HH activity and boosts cholesterol, showing CNPY4 regulates HH by altering membranes.
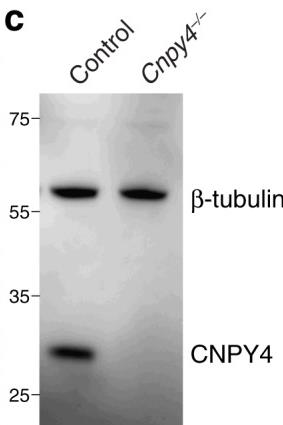
Fig1. Protein levels in lysates of MEF cells from control and mutant embryos were detected using the indicated antibodies by Western blot analysis.
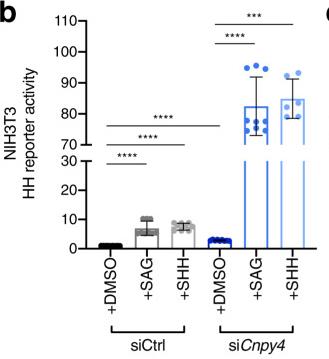
Fig2. Luciferase reporter assay in ciliated NIH3T3 cells treated with control (gray bars) or Cnpy4 (blue bars) siRNA.
Case 2: Hart BE, et al. J Biol Chem. 2012
The location of Toll-like receptors (TLRs) in cells is key to their role in sensing infections. Researchers found that the I602S change in TLR1 affects its movement to the cell surface, altering responses to certain triggers and affecting disease susceptibility. Position 602 is crucial for TLR1's cell surface presence, and a serine at this spot disrupts it. TLR chaperones, PRAT4A and PRAT4B, help or hinder this process. Increasing PRAT4A or reducing PRAT4B can fix this issue in the 602S variant. Also, IFN-γ treatment boosts TLR1 surface expression in certain monocytes, likely through PRAT4A activation. This explains how the I602S variant's trafficking is regulated differently, with distinct roles for PRAT4 proteins.
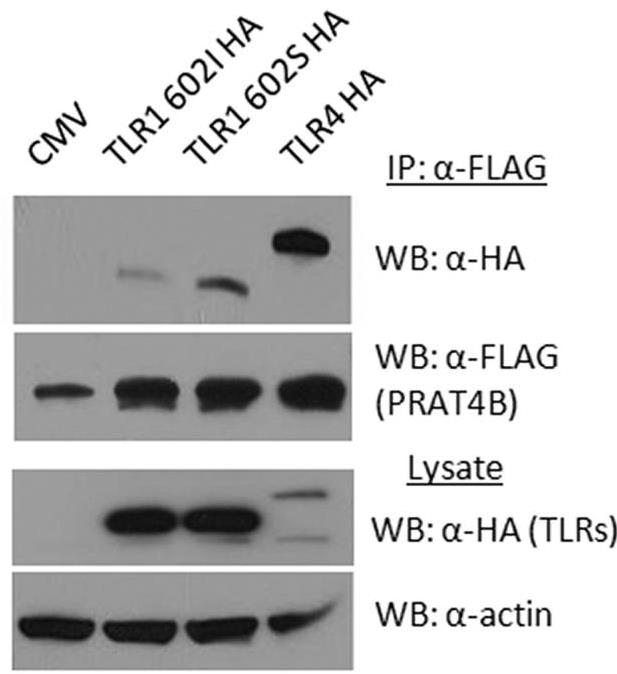
Fig1. HEK 293T cells were co-transfected with either TLR1 602I-HA, TLR1 602S-HA, or TLR4-HA, and PRAT4B-FLAG.
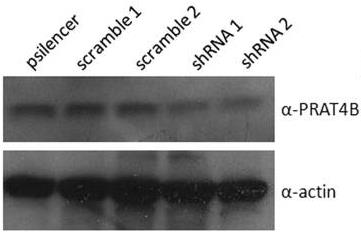
Fig2. Cell lysates from similar transient transfections were collected and probed by immunoblot for PRAT4B to assess shRNA-mediated knockdown of the chaperone.
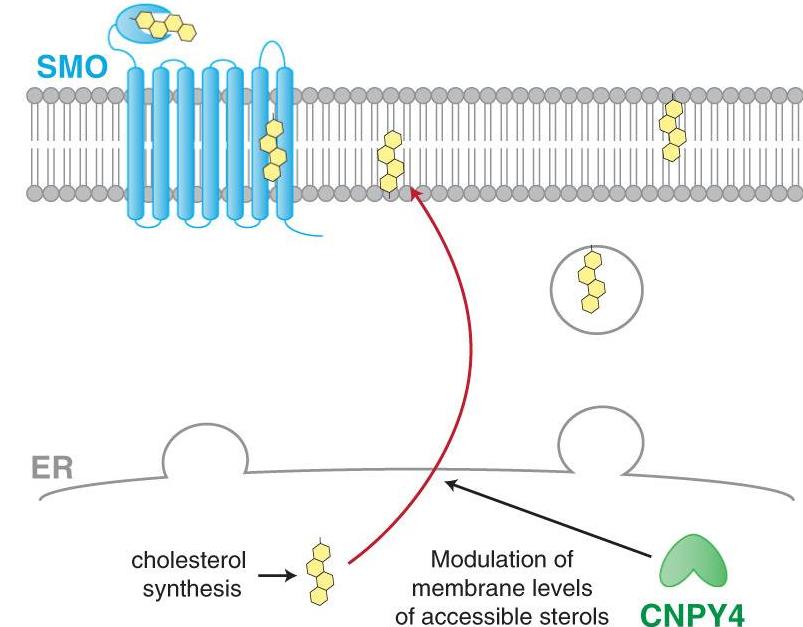
Fig1. Schematic illustrating proposed model of CNPY4 modulation of HH activation. (Ting-Ting Chang, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All CNPY4 Products
Required fields are marked with *
My Review for All CNPY4 Products
Required fields are marked with *
Inquiry Basket


