Recombinant H1N1 M1 protein(Met1-Lys252), His-tagged
| Cat.No. : | M1-416H |
| Product Overview : | Recombinant influenza A virus (A/Brevig Mission/1/ 1918(H1N1)) matrix protein 1(Q8BAC3.1) (Met1-Lys252), termed as M1, was expressed in E. coli, fused with a N-terminal polyhistidine tag. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | H1N1 |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | Met1-Lys252 |
| Form : | Lyophilized from sterile 1mM EDTA, 50mM Tris, 50mM NaCl, 5% glycerol. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant M1 subunit of influenza A H1N1 consists of 268 amino acids and has a predicted molecular mass of 30 kDa. It migrates as an approximately 35 kDa band in SDS-PAGE under reducing conditions. |
| Purity : | > 90 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
Case 1: Selzer L, et al. PLoS Biol. 2020
Many enveloped viruses, such as influenza, herpes, and coronaviruses, encode matrix proteins. In influenza viruses, the matrix protein M1 forms a multilayered structure just below the viral membrane, helping to maintain particle stability and RNA release when pH changes. However, the lack of a high-resolution structure of the complete M1 protein has prevented us from further studying how to target it for antiviral design, and it is less clear how the structure changes during pH changes when the virus enters the cell. A number of protein interfaces were identified through purification and a large number of mutation tests in the study, which are critical for the formation of multilayer helical M1 oligomers similar to those at low pH exposure. Mutations in specific amino acids revealed M1 oligomers with single-layer helical structures similar to those found in viruses that did not enter cells. Its highly ordered monolayer structure is similar to the stroma layer of a complete virus, prompting the researchers to perform structural analysis with low temperature electron microscopy, resulting in a 3.4-A resolution structure that reveals the folding details of M1 and how it is arranged in a monolayer matrix.
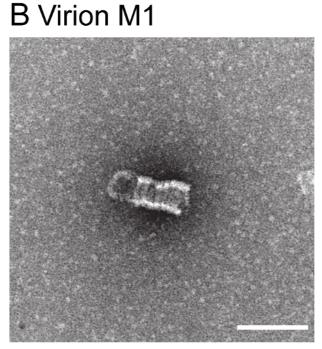
Fig1. Negative-stain EM of M1 purified from virions.
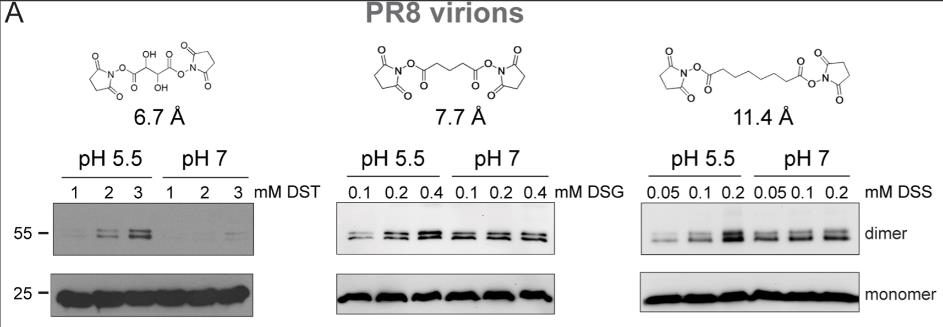
Fig2. SDS-PAGE analysis of M1 crosslinked within intact PR8 virions at increasing concentrations of DST, DSG, and DSS.
Case 2: Shtykova EV, et al. Sci Rep. 2017
Influenza A virus M1 protein is super important for the virus, but we still don't fully get how it works or its structure. So, researchers used techniques like small-angle X-ray scattering, atomic force microscopy, and zeta-potential measurements to dive into its structure and how it behaves under different pH levels. Turns out, M1 has a stable globular N-terminal domain and a flexible C-terminal extension. The N-terminal stays pretty stable from pH 4.0 to 6.8, but the C-terminal moves around a lot and its ability to join up with other proteins changes big time. Here the way these proteins bind is actually reversible and starts breaking apart when the pH hits around 6.
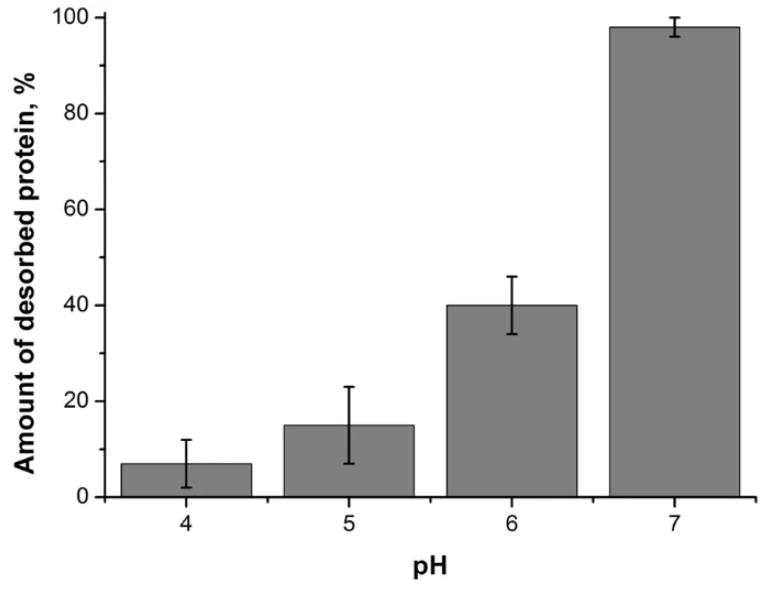
Fig1. Amount of M1 protein desorbed from a negatively charged BLM after perfusion with protein-free working buffer solution at different pH.
Fig2. Volume fraction of M1 monomers in solution depending on the protein charge.
The recombinant H1N1 M1 protein is a crucial part of the influenza A virus, especially the H1N1 type. The M1 protein's ability to adapt within a host and its role in virulence, as well as its interaction with host factors, make it essential for viral growth and spread.
In vaccine development, recombinant M1 protein shows promise as an immunogen; it can generate immune responses and offer protection against various influenza challenges in animal studies. For instance, administering it intranasally has provided protection against lethal H9N2 and offered partial protection against H1N1 and H5N1 strains. Additionally, when combined with other antigens like hemagglutinin and neuraminidase, M1 can enhance immune protection. Its role is not just limited to protection but extends to viral replication and transmission, aiding the spread of virus strains in animal models.
Recombinant H1N1 M1 protein is making waves both in research circles and the industry. In the lab, it's been a big help in diving into the nitty-gritty of how flu viruses, like H1N1, work. This kind of insight is super handy for whipping up vaccines and could lead to antiviral drugs that pack a punch. Plus, folks are using this M1 protein as a marker to cook up better virus detection techniques that are sharper and more spot-on.
Over in the industrial world, this protein has found its groove in making vaccines, especially in biotech and pharma sectors. Thanks to genetic engineering, cranking out the M1 protein is now not only faster but also friendlier on the wallet, all while keeping vaccines up to snuff. What's more, using M1 as a vaccine adjuvant helps boost the vaccine's punch while keeping side effects at bay. All these advances are giving a serious leg-up to H1N1 vaccine progress and paving the way for tackling other viruses too.
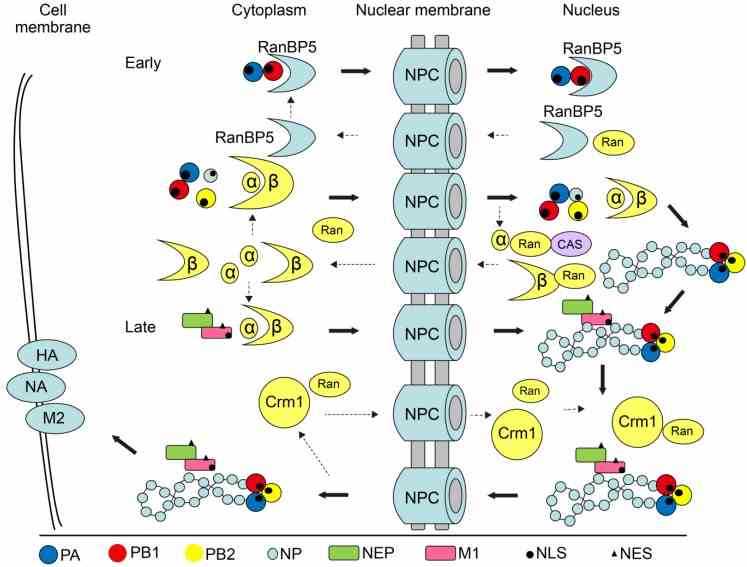
Fig1. Model of the nucleocytoplasmic shuttling of influenza virus proteins and vRNPs. (Jing Li, 2015)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All M1 Products
Required fields are marked with *
My Review for All M1 Products
Required fields are marked with *
Inquiry Basket


