Recombinant Full Length Mouse Phosphatidylinositol N-Acetylglucosaminyltransferase Subunit Y(Pigy) Protein, His-Tagged
| Cat.No. : | RFL299MF |
| Product Overview : | Recombinant Full Length Mouse Phosphatidylinositol N-acetylglucosaminyltransferase subunit Y(Pigy) Protein (P0C1P0) (1-71aa), fused to N-terminal His tag, was expressed in E. coli. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Mus musculus |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | Full Length (1-71) |
| Form : | Lyophilized powder |
| AA Sequence : | MIRSLPTMTVLIPLVSLAGLLYSASVEEGFPEGCTSASSLCFYSLLLPVTVPVYVFFHLWTWMGLKLFRHN |
| Purity : | Greater than 90% as determined by SDS-PAGE. |
| Applications : | SDS-PAGE |
| Notes : | Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week. |
| Storage : | Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles. |
| Storage Buffer : | Tris/PBS-based buffer, 6% Trehalose, pH 8.0 |
| Reconstitution : | We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference. |
| Gene Name | Pigy |
| Synonyms | Pigy; Pigyl; Phosphatidylinositol N-acetylglucosaminyltransferase subunit Y; Phosphatidylinositol-glycan biosynthesis class Y protein; PIG-Y |
| UniProt ID | P0C1P0 |
| ◆ Recombinant Proteins | ||
| PIGY-3429R | Recombinant Rhesus monkey PIGY Protein, His-tagged | +Inquiry |
| PIGY-12790M | Recombinant Mouse PIGY Protein | +Inquiry |
| PIGY-4116R | Recombinant Rat PIGY Protein, His (Fc)-Avi-tagged | +Inquiry |
| PIGY-4456R | Recombinant Rat PIGY Protein | +Inquiry |
| PIGY-5666C | Recombinant Chicken PIGY | +Inquiry |
Case 1: Murakami Y, et al. Mol Biol Cell. 2005
The human GPI-N-acetylglucosaminyltransferase (GPI-GnT) complex, which initiates the synthesis of glycosylphosphatidylinositol (GPI), requires an additional component, PIG-Y, a 71-amino acid protein with two transmembrane domains. PIG-Y interacts directly with the catalytic subunit PIG-A, indicating a regulatory role in GPI-GnT activity. The Daudi Burkitt lymphoma cell line, which lacks GPI-anchored proteins on its surface, is a PIG-Y null mutant. PIG-Y may share homology with the yeast Eri1p component of GPI-GnT and is encoded alongside another protein, PreY, by a single transcript through a process known as leaky scanning. There is no evidence of a functional connection between GPI-GnT and ras GTPases in mammalian cells, unlike in yeast.
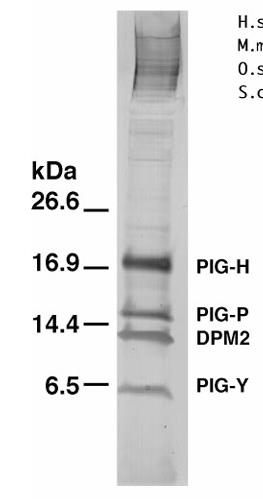
Fig1. Purification of GPI-GnT complexes.
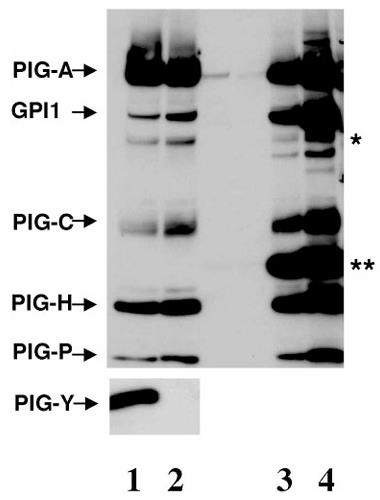
Fig2. GST-FLAG-PIG-A and the five FLAG-tagged GPI-GnT components were cotransfected with (lanes 1 and 3) or without HA-PIGY (lanes 2 and 4) into Daudi cells.
Case 2: Ilkovski B, et al. Hum Mol Genet. 2015
Proteins anchored by glycosylphosphatidylinositol (GPI) are prevalently found throughout the human body and play crucial roles in a multitude of cell surface functions. To date, a variety of mutations in genes involved in GPI biosynthesis have been identified in individuals suffering from multi-system disorders, which are collectively classified as a subset of congenital glycosylation disorders. In this study, researchers utilized whole exome sequencing on two distinct families to delve into the genetic roots of their conditions and further employed RNA and cellular analyses to explore the impact of variations found within the PIGY gene.
They discovered that both families, presenting with divergent clinical manifestations, harbored homozygous recessive mutations in the PIGY gene, which is integral to GPI biosynthesis. These PIGY gene mutations can manifest in both the coding and non-coding regions, leading to a spectrum of phenotypes. This research not only broadens the comprehension of the diverse clinical presentations and genetic underpinnings linked to deficiencies in the GPI-anchor biosynthesis pathway but also underscores the significance of examining variants identified within the 5'-untranslated regions (5'-UTR), even though these regions are commonly characterized by relatively sparse coverage in exome sequencing data.
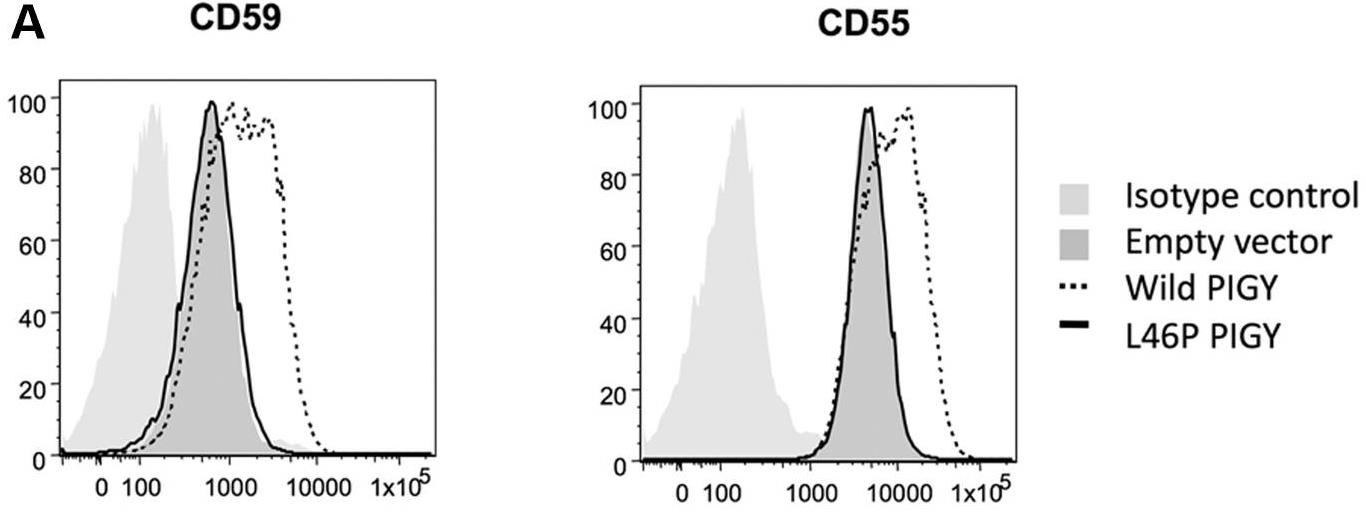
Fig1. Daudi cells were transiently transfected with normal (WT) or mutant (p.Leu46Pro) PIGY.
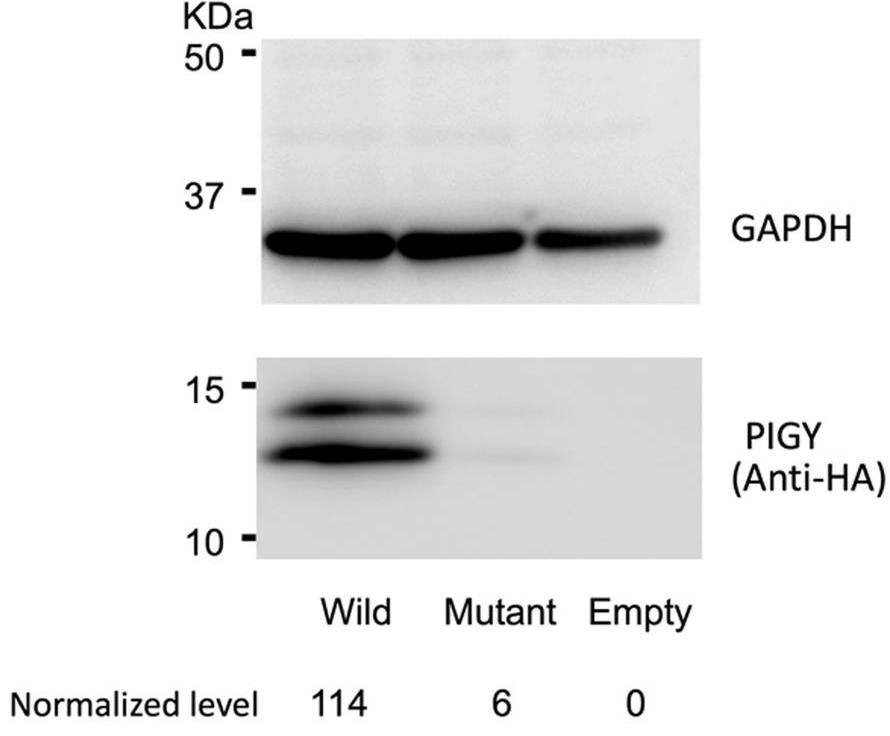
Fig2. Cell lysates prepared from Daudi cells transfected with either WT-PIGY, mutant p.Leu46Pro PIGY, and empty vector were separated by SDS-PAGE.
Pigy is part of the glycosyl phosphatidylinositol (GPI) -n-acetylglucosamine transferase (GPI-GNT) complex, which initiates the biosynthesis process of GPI. GPI is an anchoring protein that is responsible for anchoring multiple surface proteins to cell membranes and is critical for cell-cell interactions.
Due to Pigy's key role in the GPI biosynthetic pathway, it may be a target for the treatment of certain genetic diseases. For example, diseases associated with defects in GPI biosynthesis, such as multiple systemic diseases, may be treated by modulating Pigy activity or function. In addition, variations in Pigy have been linked to certain disease phenotypes, such as the syndrome of intellectual developmental disorder, suggesting a potential application of Pigy protein in disease treatment. The interaction of Pigy proteins with other components of the GPI-GNT complex is critical for GPI biosynthesis. By studying Pigy's interactions with other proteins, we can gain a deeper understanding of the regulatory mechanisms of the GPI biosynthetic pathway. In models studying specific genetic diseases, the recombinant Pigy protein may be used to simulate changes in protein function during disease states and, in turn, to develop gene therapy strategies for these diseases.
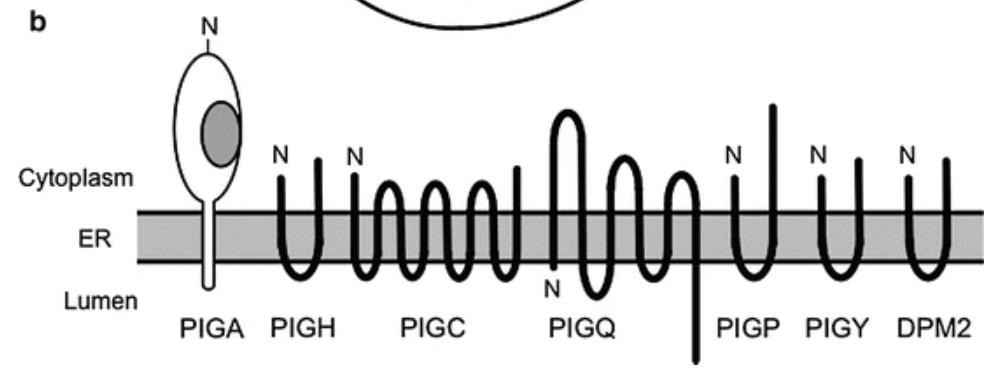
Fig1. Schematic representation of subunits of GPI-GnT. (Taroh Kinoshita, 2014)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All Pigy Products
Required fields are marked with *
My Review for All Pigy Products
Required fields are marked with *
Inquiry Basket


