Recombinant Cynomolgus FGF1 protein(Phe16-Asp155)
| Cat.No. : | FGF1-65C |
| Product Overview : | Recombinant Cynomolgus FGF1 (Phe16-Asp155) was expressed and purified in E. coli with an initial Met. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Cynomolgus |
| Source : | E.coli |
| Tag : | Non |
| Protein Length : | 16-155 a.a. |
| Form : | Lyophilized from sterile PBS, pH 7.4, 20% glycerol. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Bio-activity : | Measured in a cell proliferation assay using BALB/c 3T3 mouse fibroblasts. The ED50 for this effect is typically 0.1-0.4 ng/mL. |
| Molecular Mass : | The recombinant cyno FGF1 consists of 141 amino acids and has a calculated molecular mass of 16 kDa. It migrates as an 16 KDa band in SDS-PAGE under reducing conditions. |
| Purity : | > 95 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| ◆ Recombinant Proteins | ||
| FGF1-11H | Active Recombinant Human FGF1 Protein (Carrier-Ready-To-Use, 140 amino acid) | +Inquiry |
| FGF1-404F | Active Recombinant Human FGF1 Protein (140 aa) | +Inquiry |
| Fgf1-5666M | Recombinant Mouse Fgf1 Protein (Phe16-Asp155), C-His tagged | +Inquiry |
| FGF1-013O | Recombinant Human FGF1 | +Inquiry |
| FGF1-2528H | Recombinant Human FGF1 Protein (Phe16-Asp155) | +Inquiry |
| ◆ Native Proteins | ||
| FGF1-26203TH | Native Human FGF1 | +Inquiry |
| FGF1-33B | Active Native Bovine FGF1 | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| FGF1-6252HCL | Recombinant Human FGF1 293 Cell Lysate | +Inquiry |
Case 1: Lampart A, et al. Cell Mol Life Sci. 2023
Fibroblast growth factor 1 (FGF1) activates tyrosine kinase receptors on cell surfaces and also has intracellular effects. Here intracellular FGF1 can prevent cell death induced by various apoptotic agents, but this protection is dependent on the presence of wild-type p53. FGF1 does not protect p53-negative cells from apoptosis, and silencing p53 abolishes FGF1's protective effect. Conversely, introducing wild-type p53 into p53-negative cells restores FGF1's anti-apoptotic properties. FGF1 directly binds to the DNA binding domain (DBD) of p53, suggesting that intracellular FGF1 shields cells from apoptosis by interacting directly with p53.
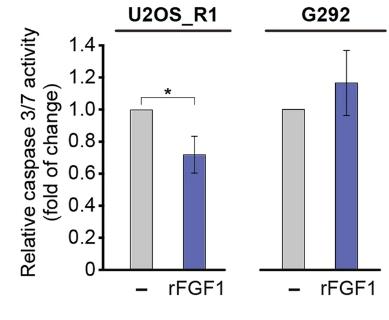
Fig1. Translocated FGF1 protects p53-positive but not p53-negative osteosarcoma cells from serum starvation-induced apoptosis.
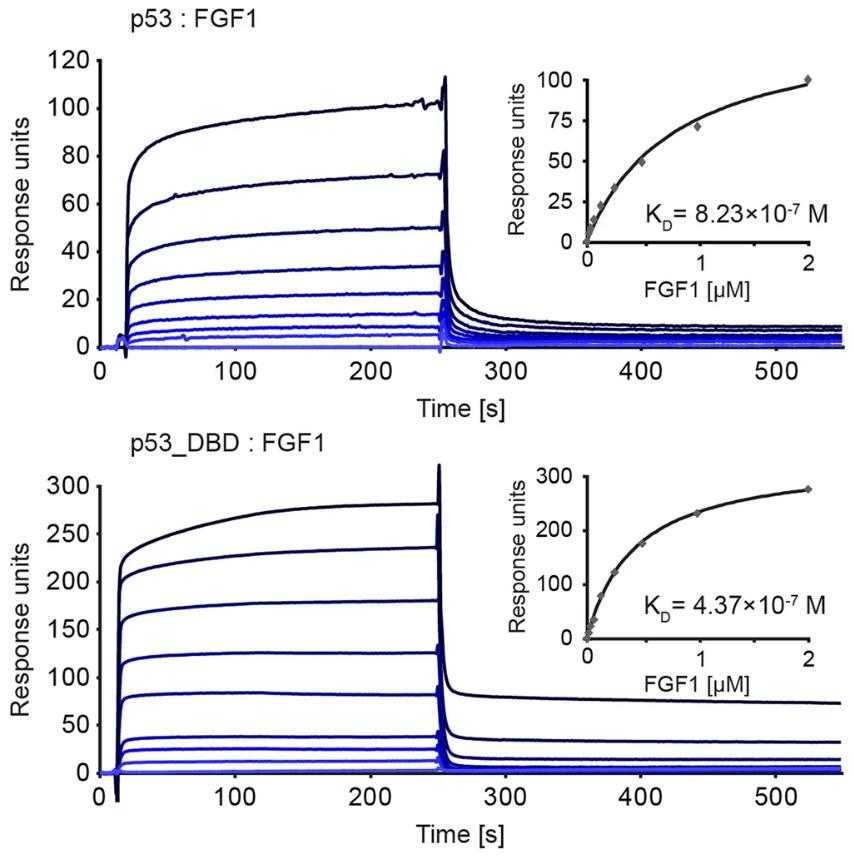
Fig2. Kinetics of p53: FGF1 and p53_DBD: FGF1 interaction assessed with SPR.
Case 2: Yu H, et al. Int J Biol Macromol. 2024
High-fat diets (HFDs) in aquaculture can lead to metabolic issues in fish, such as liver damage and glucose imbalances. The effects of fibroblast growth factor 1 (FGF1) on these issues in teleosts are not well understood. A 6-week study with rainbow trout fed an HFD and treated with recombinant FGF1 (rFGF1) showed that rFGF1 significantly lowered serum glucose and hepatic gluconeogenic enzyme activities, and increased hepatic glycogen levels. The beneficial effects were mediated by the cAMP signaling pathway and the AKT-GSK3β axis, and were associated with reduced liver inflammation and improved antioxidant status. rFGF1 also altered gut microbiota, increasing intestinal Lactobacillus and short-chain fatty acid levels. This research provides insights into the role of rFGF1 in regulating glucose metabolism and reducing inflammation in HFD-fed fish, potentially offering a strategy to manage metabolic disorders in carnivorous fish.
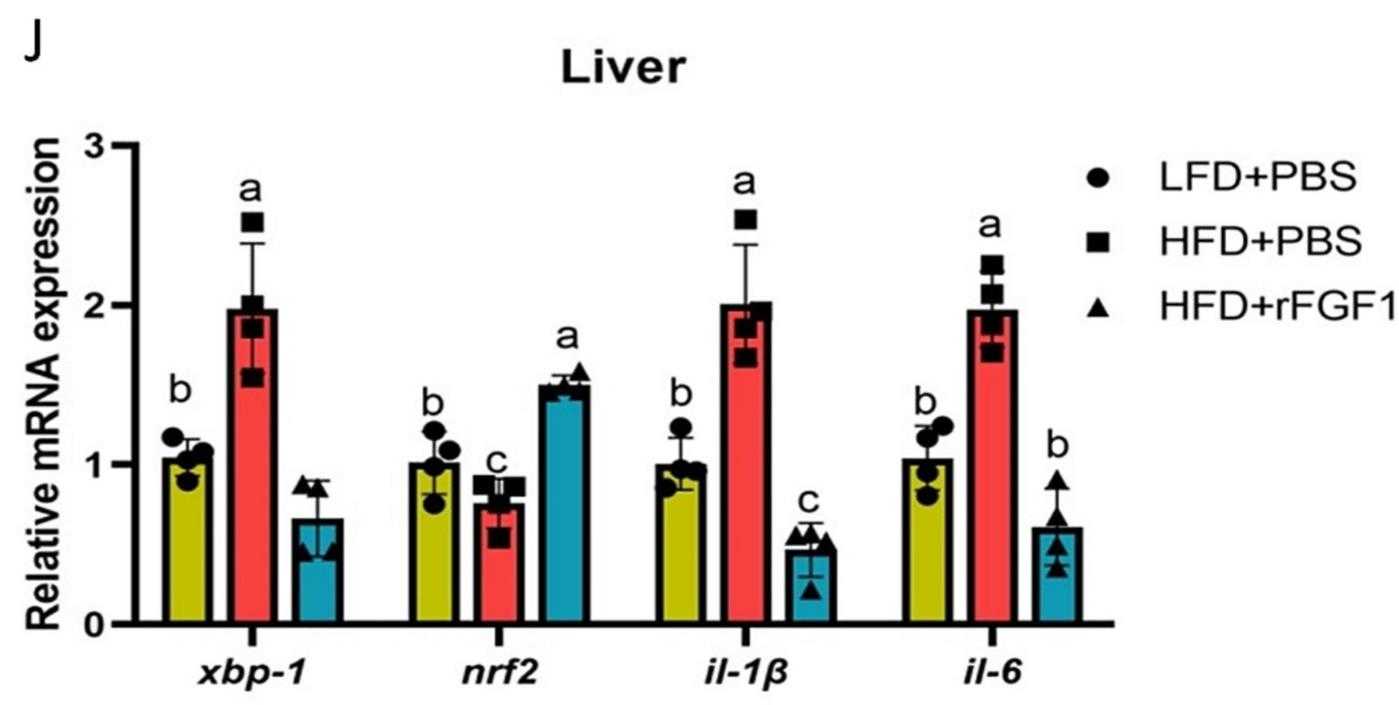
Fig1. The mRNA expression of inflammation-related genes in the liver.
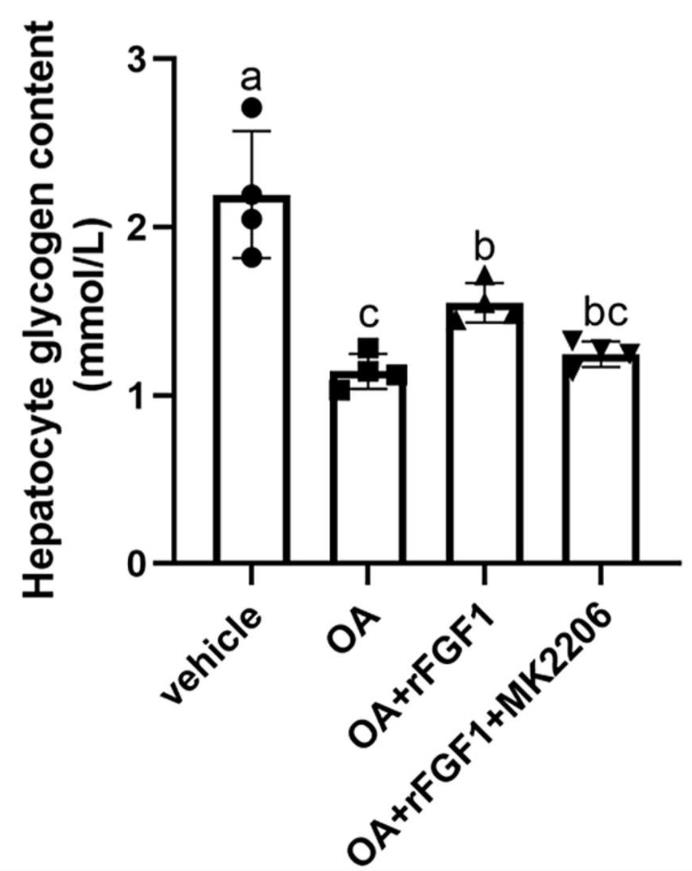
Fig2. Hepatocyte glycogen content.
Recombinant Cynomolgus FGF1 protein, a member of the fibroblast growth factor family, has a multitude of applications in life sciences and medical research. It plays a significant role in metabolic regulation, disease management, and cellular protection. In metabolic research, FGF1 is crucial for controlling glucose levels and modulating immune reactions, making it a potential therapeutic target for conditions like diabetes and non-alcoholic fatty liver disease. It has also been shown to improve glucose homeostasis and reduce inflammatory responses in models such as rainbow trout fed a high-fat diet, indicating its potential in managing metabolic disorders.
In cellular protection, intracellular FGF1 has been demonstrated to protect cells from apoptosis by directly interacting with p53, a protein central to cell cycle regulation and apoptosis. This interaction is critical for the anti-apoptotic effect of intracellular FGF1, suggesting its potential role in cancer therapy and neurodegenerative disease treatment where apoptosis regulation is crucial.
Furthermore, FGF1 is involved in the proliferation and differentiation of various cell types, including muscle satellite cells, where it promotes mitochondrial fission and proliferation under serum-free conditions. This highlights its potential in tissue engineering and cellular agriculture, such as in the production of cultured meat.
In summary, recombinant Cynomolgus FGF1 protein is a versatile molecule with implications in metabolic disease management, cell protection, tissue engineering, and therapeutic development, making it an important asset in the field of biomedicine.
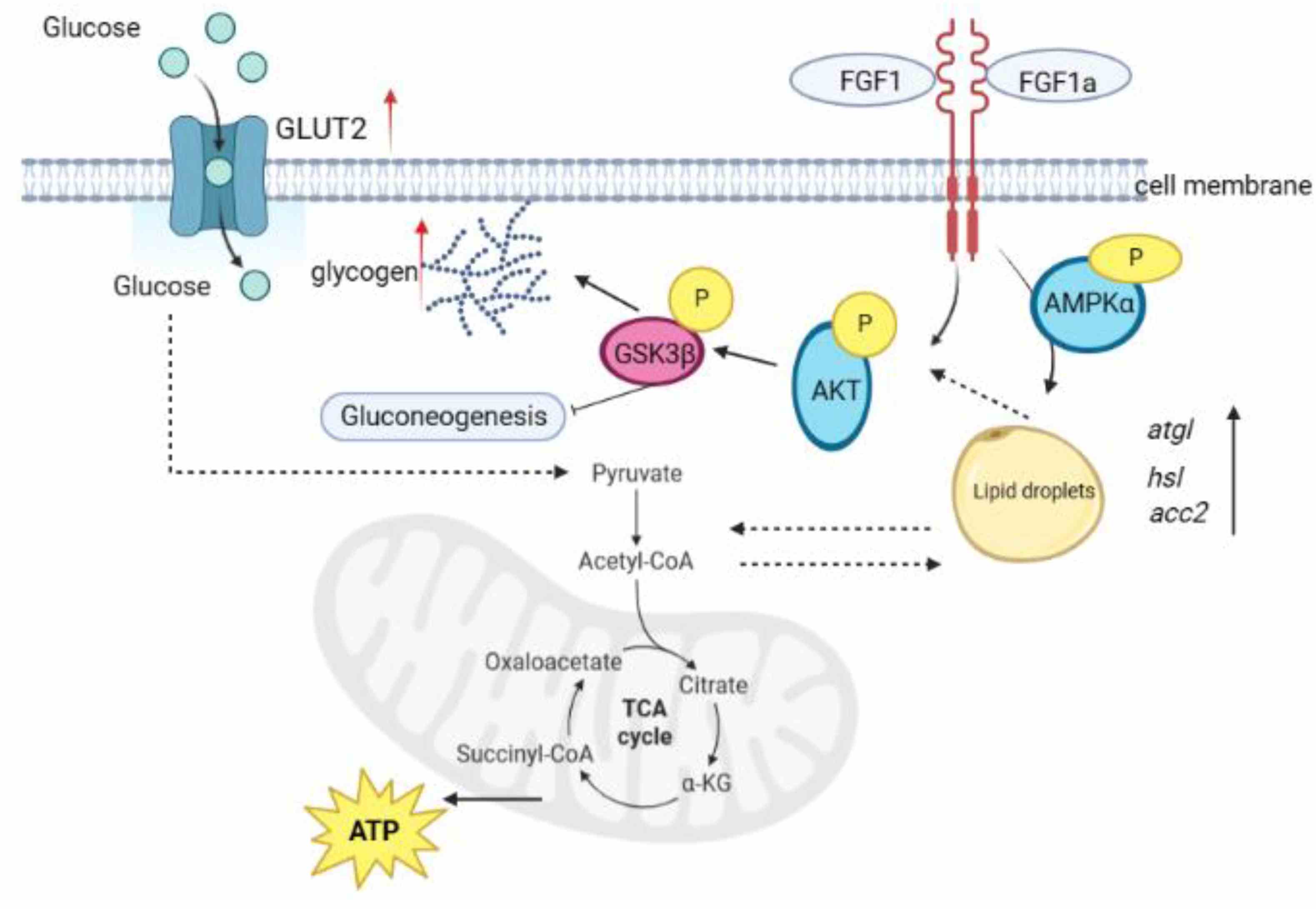
Fig1. Summary of the results showing rainbow trout FGF1 and FGF1a improved lipid metabolism disorders in juvenile rainbow trout. (Huixia Yu, 2024)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All FGF1 Products
Required fields are marked with *
My Review for All FGF1 Products
Required fields are marked with *
Inquiry Basket


