Native Human Apo Transferrin Protein, Tag Free
| Cat.No. : | TF-62H |
| Product Overview : | Native human Apo Transferrin Protein without Tag was purified from human serum. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | Serum |
| Tag : | Non |
| Protein Length : | 1-698aa |
| Description : | Transferrins are iron binding transport proteins which can bind two Fe3+ ions in association with the binding of an anion, usually bicarbonate. It is responsible for the transport of iron from sites of absorption and heme degradation to those of storage and utilization. Serum transferrin may also have a further role in stimulating cell proliferation. |
| Tag : | Non |
| Form : | Sterile Filtered White lyophilized (freeze-dried) powder |
| Molecular Mass : | 77 kDa |
| Purity : | Greater than 98.0% as determined by coomassie blue stained SDS-PAGE and Cellulose Acetate electrophoresis. |
| Notes : | Our products are furnished for LABORATORY RESEARCH USE ONLY. The product may not be used as drugs, agricultural or pesticidal products, food additives or household chemicals. |
| Storage : | Store the lyophilized Apo Transferrin between 2-8 centigrade, do not freeze. Upon reconstitution Apo Transferrin should be stored at 4 centigrade between 2-7 days and for future use below -18 centigrade. Please prevent freeze-thaw cycles. |
| Storage Buffer : | The protein was lyophilized from 20mM NH4HC03 solution. May contain traces of buffer salts. |
| Solubility : | It is recommended to reconstitute the lyophilized Apo Transferrin in sterile 18MΩ-cm H2O not less than 100 μg/mL, which can then be further diluted to other aqueous solutions. |
| Shipping : | Room temperature. |
| Human Virus Test : | Plasma from each donor has been tested and found negative for antibody to HIV-1, HIV-2, HCV, HBSAG and Syphilis. |
| Iron Content : | The Iron content was estimated by ICP and was found to be <6 ppm. |
| References : | 1. Src-Mediated Phosphorylation of Dynamin and Cortactin Regulates the "Constitutive" Endocytosis of Transferrin. Molecular and cellular biology 30.3 (2010): 781-792. |
| Gene Name | TF transferrin [ Homo sapiens (human) ] |
| Official Symbol | TF |
| Synonyms | TF; transferrin; serotransferrin; PRO1557; PRO2086; siderophilin; beta-1 metal-binding globulin; TFQTL1; DKFZp781D0156 |
| Gene ID | 7018 |
| mRNA Refseq | NM_001063 |
| Protein Refseq | NP_001054 |
| MIM | 190000 |
| UniProt ID | P02787 |
| ◆ Recombinant Proteins | ||
| Trf-148M | Recombinant Mouse Trf Protein, His-tagged | +Inquiry |
| TF-1589C | Recombinant Canine TF protein, His-tagged | +Inquiry |
| TF-150P | Recombinant Pig TF Protein, His-tagged | +Inquiry |
| TF-3192H | Recombinant Human TF, GST-tagged | +Inquiry |
| Trf-8683M | Recombinant Mouse Trf protein, His-tagged | +Inquiry |
| ◆ Native Proteins | ||
| TF-31158TH | Native Human TF | +Inquiry |
| TF-172S | Native Sheep transferrin | +Inquiry |
| TF-323H | Native Human Transferrin Fluorescein | +Inquiry |
| Trf-4782M | Native Mouse Transferrin | +Inquiry |
| Tf-392R | Native Rat Transferrin | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| TF-1554SCL | Recombinant Sus scrofa (Pig) TF cell lysate | +Inquiry |
| TF-2542HCL | Recombinant Human TF cell lysate | +Inquiry |
| TF-001RCL | Recombinant Rat TF cell lysate | +Inquiry |
| TF-1208MCL | Recombinant Mouse TF cell lysate | +Inquiry |
Case 1: Tang X, et al. Cell Res. 2020
The balance of blood coagulation relies on the interactions among clotting factors at different physiological concentrations. Coagulation proteases are usually at lower concentrations compared to their inactivator, antithrombin (AT). Our study reveals that transferrin, typically found at around 40 μM in plasma, interacts with fibrinogen, thrombin, factor XIIa (FXIIa), and AT to help maintain this balance. In a healthy state, transferrin binds to fibrinogen at a 4:1 molar ratio. However, in atherosclerosis, elevated transferrin levels enhance thrombin and FXIIa activity while inhibiting AT's effect, leading to excessive blood clotting. In mice, increased transferrin worsens atherosclerosis, but reducing transferrin levels through shRNA, antibodies, or peptides blocking its interaction with thrombin/FXIIa can ease the condition.
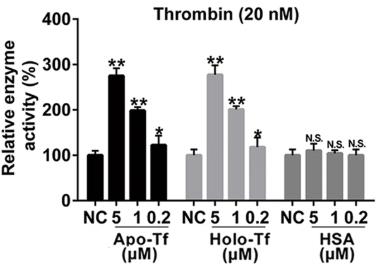
Fig1. Potentiating effects of both apo- and holo-transferrin on thrombin.
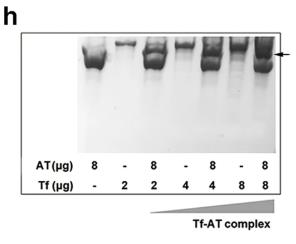
Fig2. Native gel shift analysis of interaction between transferrin and AT.
Case 2: Baringer SL, et al. J Biomed Sci. 2023
Apo-transferrin (without iron) and holo-transferrin (with iron) play key roles in controlling how iron is taken up by the brain at the blood-brain barrier. Apo-Tf signals low iron levels, prompting iron release, while holo-Tf indicates enough iron, so it curbs additional release. Iron gets exported via ferroportin, aided by hephaestin. Although previously unclear, our study uncovers how these transferrin forms affect iron release. Using tests like co-immunoprecipitation with cultured cells, we found that holo-Tf triggers ferroportin's internalization and engages directly with it, whereas apo-Tf pairs with hephaestin. Interestingly, high levels of hepcidin can break the holo-Tf and ferroportin connection but don't affect the apo-Tf and hephaestin bond, mainly because hepcidin speeds up ferroportin internalization more effectively than holo-Tf can.
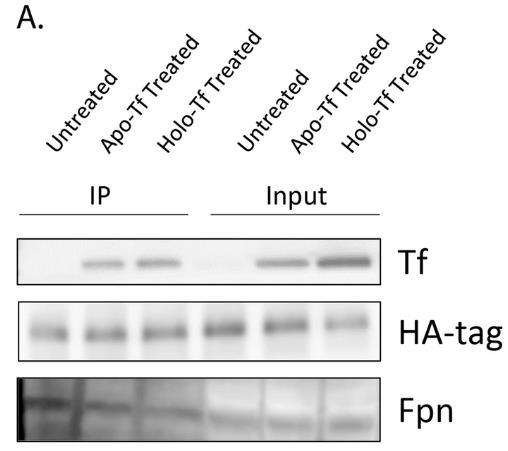
Fig1. Co-IP of HA-Fpn shows that both apo- and holo-Tf are pulled down along with the Fpn complex.
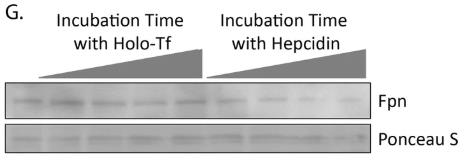
Fig2. Hepcidin reduces membrane Fpn at a faster rate than holo-Tf.
Native human Apo Transferrin Protein is a key player in both research and industrial applications due to its essential role in iron metabolism. As the primary glycoprotein in serum, it is responsible for ferrying iron from storage sites to tissues, ensuring stable iron levels across biological fluids. In cell cultures, Apo Transferrin is indispensable for cultivating various cell types, including primary human pancreatic endothelial cells, non-tumor esophageal cells, and immortalized colon epithelial cells. Beyond its cell culture applications, it's also utilized in crystallography studies as a precursor to purified human Apo Transferrin.
In the realm of biotechnology and medicine, Apo Transferrin has sparked innovation in therapeutic strategy development. It serves as a serum substitute in serum-free media, enhancing cell culture techniques. Additionally, it's a promising component in drug delivery and targeting systems, paving the way for novel medical treatments. Overall, Native human Apo Transferrin Protein is a cornerstone of biomedical research, cell culture, and drug development, crucial for advancing various scientific and medical fields.
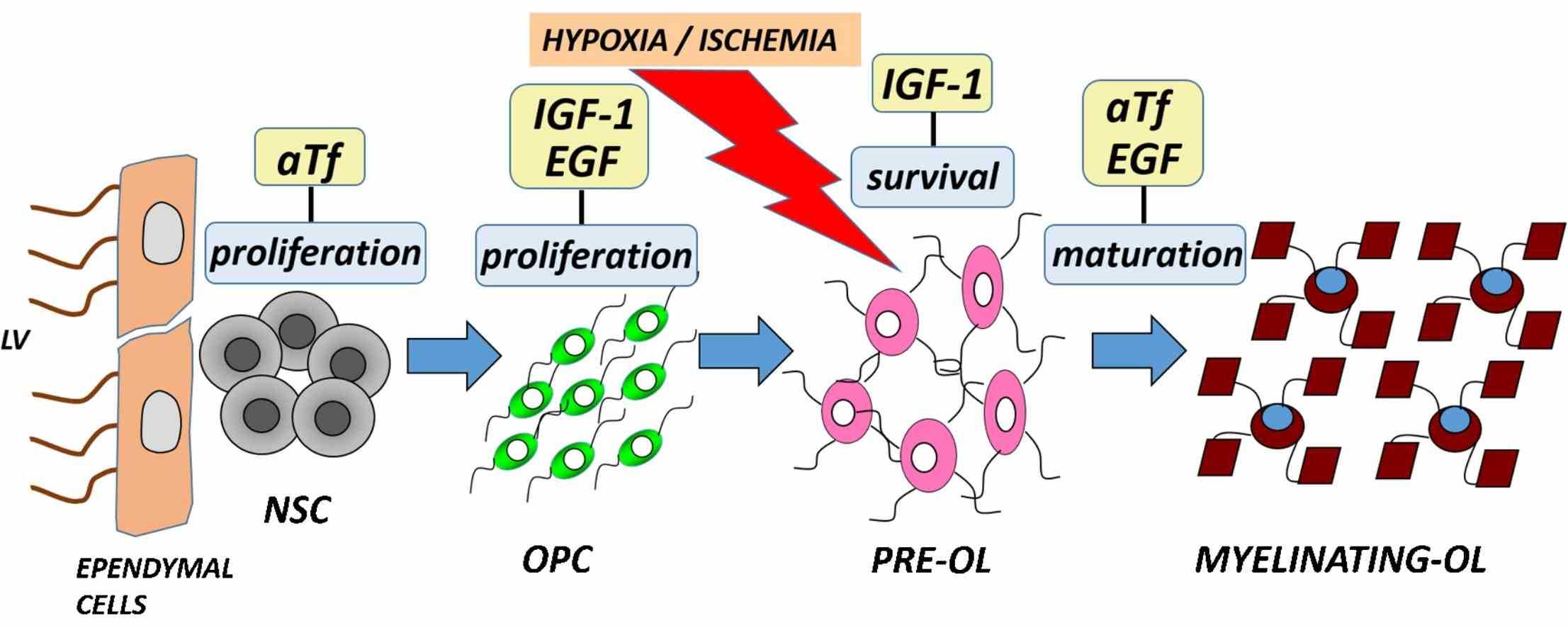
Fig1. INA of growth factors and aTf can be used in combination in clinical treatment in order to protect and repair the hypoxic-ischemic brain. (M Guardia Clausi, 2016)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All TF Products
Required fields are marked with *
My Review for All TF Products
Required fields are marked with *
Inquiry Basket


