Recombinant streptavidin
| Cat.No. : | Streptavidin-501 |
| Product Overview : | Recombinant streptavidin from Streptomyces avidinii, was produced in Escherichia coli. |
| Availability | April 21, 2025 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Streptomyces avidinii |
| Source : | E.coli |
| Tag : | Non |
| Description : | Streptavidin is a tetrameric protein composed of identic subunits. Each subunit binds one biotin molecule with a KD of ~1×10^-15M. The preparation contains an N- and C-terminal shortened variant (core streptavidin) with improved properties concerning homogeneity, solubility, resistance towards proteolytic degradation and accessibility of the biotin binding pocket as compared to native streptavidin. |
| Form : | Lyophilized in 10 mM potassium phosphate buffer pH 6.5 |
| Molecular Mass : | ~53,000 per tetramer. |
| AA Sequence : | MAEAGITGTW YNQLGSTFIV TAGADGALTG TYESAVGNAE SRYVLTGRYD SAPATDGSGT ALGWTVAWKN NYRNAHSATT WSGQYVGGAE ARINTQWLLT SGTTEANAWK STLVGHDTFT KVKPSAAS |
| Endotoxin : | Less than 1EU/μg of rStreptavidin as determined by LAL method. |
| Purity : | >95% by SDS-PAGE |
| Unit Definition : | >17 U/mg (one unit binds 1 μg D-biotin at pH 8.9) |
| Usage : | This material is for research, laboratory or further evaluation purposes. NOT FOR HUMAN USE. |
| Stability : | 2 years after shipping |
| Storage : | -20 centigrade. Avoid repeated freeze/thaw cycles. |
| Reconstitution : | Dissolve with double distilled water |
| Shipping : | Room temperature |
| Publications : |
Se-SAD serial femtosecond crystallography datasets from selenobiotinyl-streptavidin. (2017)
Selenium single-wavelength anomalous diffraction de novo phasing using an X-ray-free electron laser. (2016)
A Brief Tour of the Apo-Streptavidin Structures at the SSRL and LCLS Facilities (2021)
Cooperative Allostery and Structural Dynamics of Streptavidin at Cryogenic- and Ambient-temperature (2021)
|
| ◆ Recombinant Proteins | ||
| Streptavidin-526 | Recombinant Streptomyces avidinii Streptavidin Protein | +Inquiry |
| Streptavidin-1030N | Recombinant Streptavidin protein | +Inquiry |
| Streptavidin-390S | Recombinant Streptomyces Avidinii Streptavidin, His-tagged | +Inquiry |
| Streptavidin-5117S | Active Recombinant Streptomyces avidinii Streptavidin | +Inquiry |
| Streptavidin-2939S | Core Streptavidin | +Inquiry |
| ◆ Native Proteins | ||
| Streptavidin-016 | Native Streptomyces avidinii Streptavidin, Gold conjugated | +Inquiry |
| Streptavidin-24 | Streptavidin | +Inquiry |
Case 1: Yoon CH, et al. Sci Data. 2017
This study provides a detailed description of selenobiotinyl-streptavidin (Se-B SA) co-crystal datasets recorded using the Coherent X-ray Imaging (CXI) instrument at the Linac Coherent Light Source (LCLS) for selenium single-wavelength anomalous diffraction (Se-SAD) structure determination. The dataset was collected at three different transmissions (100, 50, and 10%) using a serial sample chamber setup which allows for two sample chambers, a front chamber and a back chamber, to operate simultaneously. Diffraction patterns from Se-B SA were recorded to a resolution of 1.9 Å. The dataset is publicly available through the Coherent X-ray Imaging Data Bank (CXIDB) and also on LCLS compute nodes as a resource for research and algorithm development.
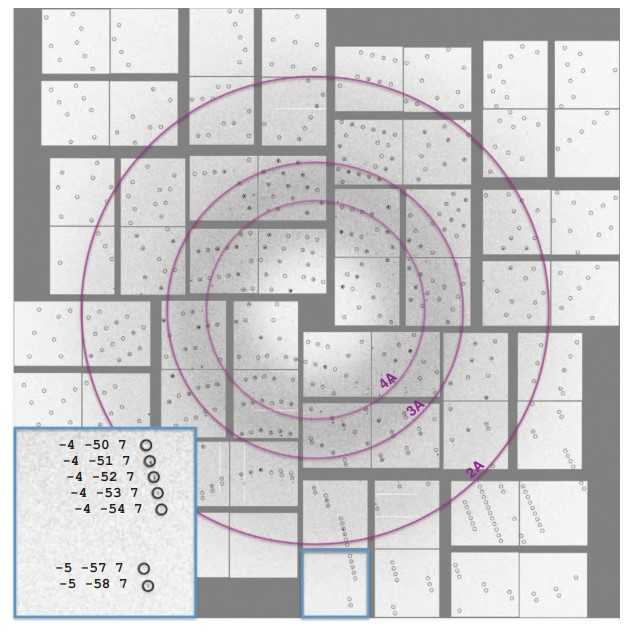
Fig1. Diffraction pattern with predicted Bragg peak positions.
Case 2: Hunter MS, et al. Nat Commun. 2016
To date, X-ray crystallography has been the predominant method used for macromolecular structure determination. However, challenges exist when solving structures with X-rays, including the phase problem and radiation damage. De novo phasing at X-ray FELs has proven challenging due in part to per-pulse variations in intensity and wavelength. Here researchers report the solution of a selenobiotinyl-streptavidin structure using phases obtained by the anomalous diffraction of selenium measured at a single wavelength (Se-SAD) at the Linac Coherent Light Source. The results demonstrate Se-SAD, routinely employed at synchrotrons for novel structure determination, is now possible at X-ray FELs.
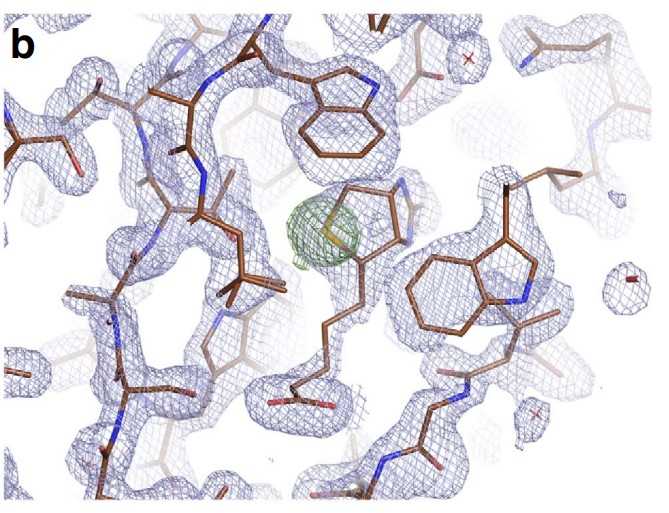
Fig1. Electron density of the final, refined structure.
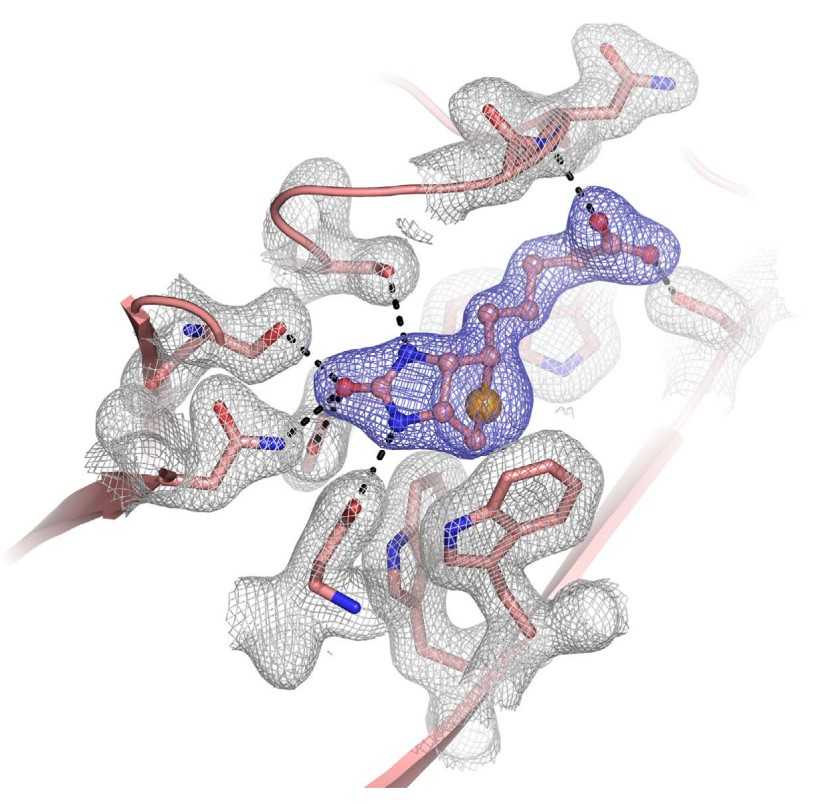
Fig2. Electron-density map of the selenobiotin-binding site.
Recombinant streptavidin protein, which is a biotin-binding protein derived from Streptomyces avidinii, has a wide range of applications in various fields. Some of the key applications of recombinant streptavidin protein include:
1. Affinity chromatography: Streptavidin is commonly used in affinity chromatography to purify biotinylated proteins, peptides, nucleic acids, and other molecules of interest. The high affinity between streptavidin and biotin allows for efficient and specific purification of target molecules.
2. Immunoassays: Streptavidin is often used as a detection reagent in immunoassays, such as enzyme-linked immunosorbent assays (ELISA) and Western blotting. It can be conjugated to enzymes, fluorophores, or other labels for the detection of specific antigens or antibodies.
3. Protein labeling and detection: Recombinant streptavidin can be conjugated to various probes, such as fluorophores, enzymes, or nanoparticles, for protein labeling and detection in applications such as immunofluorescence microscopy and flow cytometry.
4. Cell imaging and tracking: Streptavidin conjugated to fluorescent dyes or quantum dots can be used for cell imaging and tracking studies. This allows researchers to visualize and monitor the movement of specific cells or cellular structures in real-time.
5. Drug delivery: Recombinant streptavidin can be used to functionalize drug-loaded nanoparticles or liposomes for targeted drug delivery. The high binding affinity of streptavidin-biotin interaction ensures specific targeting of drug-loaded carriers to cells or tissues expressing biotinylated receptors.
6. Biosensors: Streptavidin-coated surfaces or nanoparticles can be used in biosensor platforms for the detection of various analytes, such as proteins, nucleic acids, and small molecules. The specific binding of biotinylated ligands to streptavidin allows for sensitive and selective detection of target molecules.
7. Molecular biology research: Recombinant streptavidin is widely used in molecular biology research for applications such as protein purification, protein-protein interaction studies, and nucleic acid detection. It provides a versatile tool for various experimental techniques.
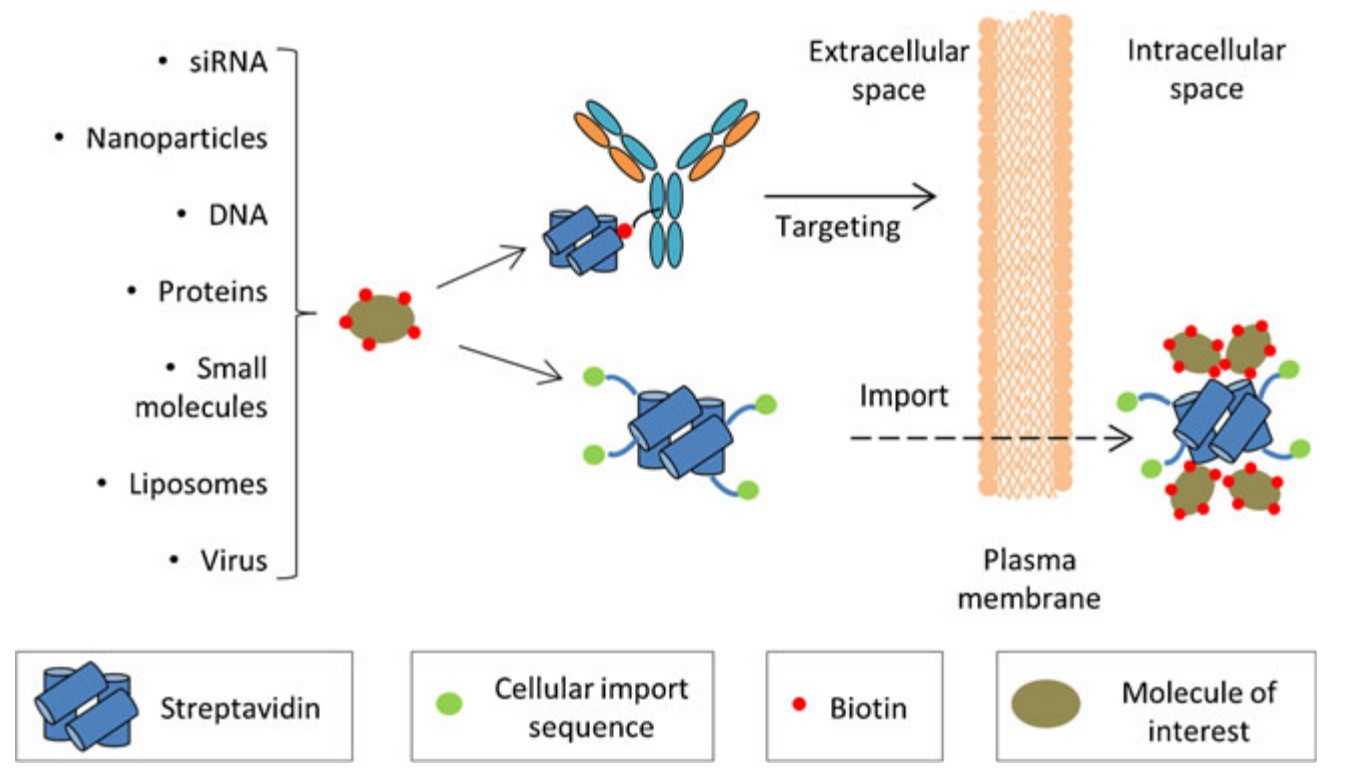
Fig1. Streptavidin-based strategies for targeted drug delivery. (Christopher M Dundas, 2013)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All Streptavidin Products
Required fields are marked with *
My Review for All Streptavidin Products
Required fields are marked with *
Inquiry Basket


