Recombinant Human CALML5 protein(Met1-Glu146), His&GST-tagged
| Cat.No. : | CALML5-3928H |
| Product Overview : | Recombinant Human CALML5 (AAH39172.1) (Met 1-Glu 146) was expressed in E. coli, fused with the N-terminal polyhistidine-tagged GST tag at the N-terminus. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | GST&His |
| Protein Length : | 1-146 a.a. |
| Form : | Lyophilized from sterile 20mM Tris, 150mM NaCl, 1mM DTT, 0.5mM GSH, 10% glycerol, pH 7.8 Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human CALML5/GST chimera consists of 388 amino acids and has a calculated molecular mass of 44.2 kDa. It migrates as an approximately 43 kDa band in SDS-PAGE under reducing conditions. |
| Purity : | > 92 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | CALML5 calmodulin-like 5 [ Homo sapiens ] |
| Official Symbol | CALML5 |
| Synonyms | CALML5; calmodulin-like 5; calmodulin-like protein 5; calmodulin like skin protein; CLSP; calmodulin-like skin protein; |
| Gene ID | 51806 |
| mRNA Refseq | NM_017422 |
| Protein Refseq | NP_059118 |
| MIM | 605183 |
| UniProt ID | Q9NZT1 |
| ◆ Recombinant Proteins | ||
| CALML5-3241H | Recombinant Human CALML5 protein, His-tagged | +Inquiry |
| CALML5-369H | Recombinant Human CALML5 Protein, His-tagged | +Inquiry |
| CALML5-0818H | Recombinant Human CALML5 Protein (Met1-Glu146), His tagged | +Inquiry |
| CALML5-609R | Recombinant Rhesus monkey CALML5 Protein, His-tagged | +Inquiry |
| CALML5-0307H | Recombinant Human CALML5 Protein, GST-Tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| CALML5-7886HCL | Recombinant Human CALML5 293 Cell Lysate | +Inquiry |
Case 1: Sun BK, et al. Genes Dev. 2015
When epidermal progenitor cells move outward, they trigger numerous differentiation genes, but not all the regulators needed for this are known. Through laser capture microdissection and RNA sequencing, calmodulin-like 5 (CALML5) emerged as highly expressed in the outer differentiating epidermis. Its mRNA levels rise due to the ZNF750 transcription factor and are stabilized by the long noncoding RNA TINCR. Knocking out CALML5 disrupts differentiation, eliminates keratohyalin granules, and harms the skin's barrier. Mass spectrometry revealed that CALML5 binds to the protein SFN (also known as stratifin or 14-3-3σ). CALML5 teams up with SFN in the upper layers of the epidermis, jointly controls a portion of late differentiation genes, and influences SFN's interactions with its partners. This ZNF750-TINCR-CALML5-SFN network is crucial for proper skin differentiation.
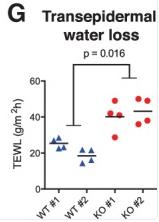
Fig1. Transepidermal water loss of CALML5 wild-type and knockout xenografts on live mice.

Fig2. Far Western blot with recombinant CALML5 or glutathione S-transferase (GST) control, probed with recombinant SFN protein.
Case 2: Méhul B, et al. J Biol Chem. 2000
Using a technique called two-dimensional gel electrophoresis, researchers explored protein extracts from human skin's stratum corneum. This investigation uncovered a new calcium-binding protein. By using reverse transcriptase-PCR on keratinocyte libraries from both growing and differentiated skin cells, they managed to clone the full-length cDNA encoding this novel protein, composed of 146 amino acids, tied to the calmodulin family. Named calmodulin-like skin protein (CLSP), this protein is notably abundant in skin where it relates closely to keratinocyte differentiation. When expressed in E. coli, the resulting recombinant protein, rCLSP, demonstrated its ability to bind calcium, much like calmodulin, and likely interacts with target proteins. Tests revealed that transglutaminase 3, an enzyme pivotal in skin cell maturation, binds to CLSP, suggesting CLSP's significant role in skin development.
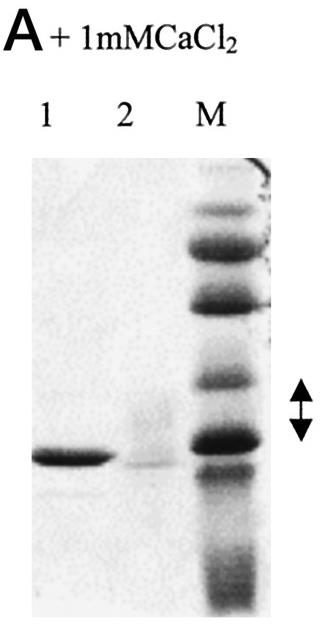
Fig1. rCLSP (1) and CaM (2) were run on a 15% acrylamide SDS gel in the presence of calcium.
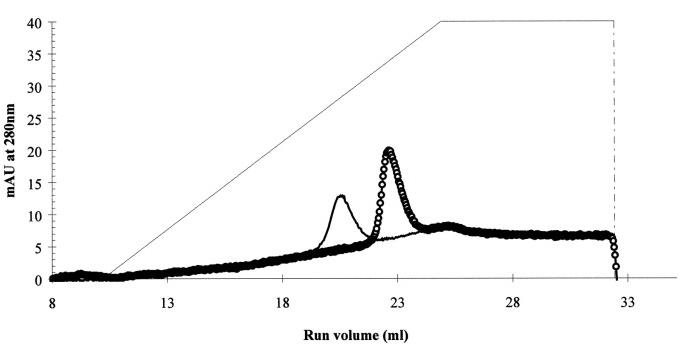
Fig2. Phenyl-Superose chromatography of rCLSP and CaM from hog brain, eluted with an ethylene glycol gradient.
Human CALML5 is part of the Calmodulin family and is known for binding calcium. This protein is key in sending signals within cells, mainly affecting skin processes. It's most active in the outer skin layer (epidermis), where it helps with cell growth, making cells distinct, and the natural cycle of cell death. Since CALML5 is mainly found in maturing skin cells, researchers are keen on exploring its function in skin development, especially its interaction with transglutaminase 3, crucial for skin cell maturation. This makes it a frequent subject in lab studies using techniques like ELISA and Western blot to study its role in various tissues and diseases.
In industrial settings, recombinant Human CALML5 is leveraged for drug discovery and development. Expressed in E. coli with an N-terminal His-GST tag, it's a valuable tool for probing cell signaling pathways and skin disorders. Additionally, it supports the development of diagnostic tests and antibodies to track CALML5 levels in biological samples, which is essential for understanding its roles in health and disease. The consistent production of this recombinant protein helps create standardized research reagents, ensuring that scientific experiments are reliable and reproducible.
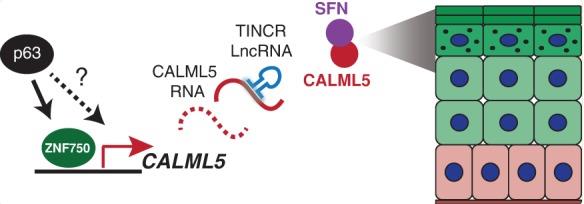
Fig1. During epidermal differentiation, CALML5 is induced by ZNF750, is post-transcriptionally stabilized by TINCR. (Bryan K Sun, 2015)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All CALML5 Products
Required fields are marked with *
My Review for All CALML5 Products
Required fields are marked with *
Inquiry Basket


