Blue Fluorescent Protein
| Cat.No. : | BFP-2 |
| Product Overview : | The recombinant Blue Fluorescent Protein (BFP) is expressed and purified from transformed E. coli using a method that ensures high purity and maximal BFP fluorescence. The protein is a 29 kDa monomer with 259 amino acids, pI: 6.17. Ex.= 308-383 nm; Em.= 440-447 nm. The protein is engineered with 6xHis-tag on the N-terminal, which can be used for detection with anti-His-Tag antibody, or protein removal by using Ni++ beads. BFP protein |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Source : | E.coli |
| Tag : | His |
| Form : | Freeze Dried |
| Molecular Mass : | 29.0 kDa |
| AA Sequence : | MGSSHHHHHHSSGLVPRGSHMVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICTTGKLPVPWPTLVTTLSHGVQCFSRYPDHMKQHDFFKSAMPEGYVQERTIFFKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNFNSHNVYIMADKQKNGIKANFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDSHYLSTQSALSKDPNEKRDHMV LLEFVTAAGITLGMDELYK |
| Endotoxin : | <0.1> |
| Purity : | Purity by SDS-PAGE: ≥97%Purity by HPLC: ≥97% |
| Usage : | For Research Use Only! Not to be used in humans. |
| Reconstitution : | Reconstitute with dH?O to 1 mg/ml |
| Shipping : | Gel pack |
| ◆ Recombinant Proteins | ||
| Blue-193 | Recombinant Blue Fluorescent Protein (Full length), N-His tagged | +Inquiry |
| BFP-001E | Recombinant Blue Fluorescent Protein, His-tagged | +Inquiry |
Case 1: Grillová L, et al. Mol Microbiol. 2024
The newly developed technique for culturing and genetically manipulating Treponema pallidum subsp. pallidum, T. pallidum has greatly advanced syphilis research, making it possible to evaluate the effects of antibiotics in the laboratory and control for differences in gene expression. As well as generating loss-of-function mutants to assess the contribution of specific gene loci to the virulence of T. pallidum. Building on this progress, researchers genetically engineered the T. pallidum SS14 strain to express red-shifted green fluorescent protein (GFP) and engineered Sf1Ep cells to express mCherry and blue fluorescent protein (BFP) to enhance visualization. These new resources improve the possibilities of T. pallidum applications based on microscopy and cell sorting to better capture physical interactions between host and pathogen, among others. In order to bring the overall knowledge of T. pallidum's biology and the pathogenesis of syphilis to the level of other bacterial pathogens, including spirochaeta, new tools and resources need to be continuously developed and shared.
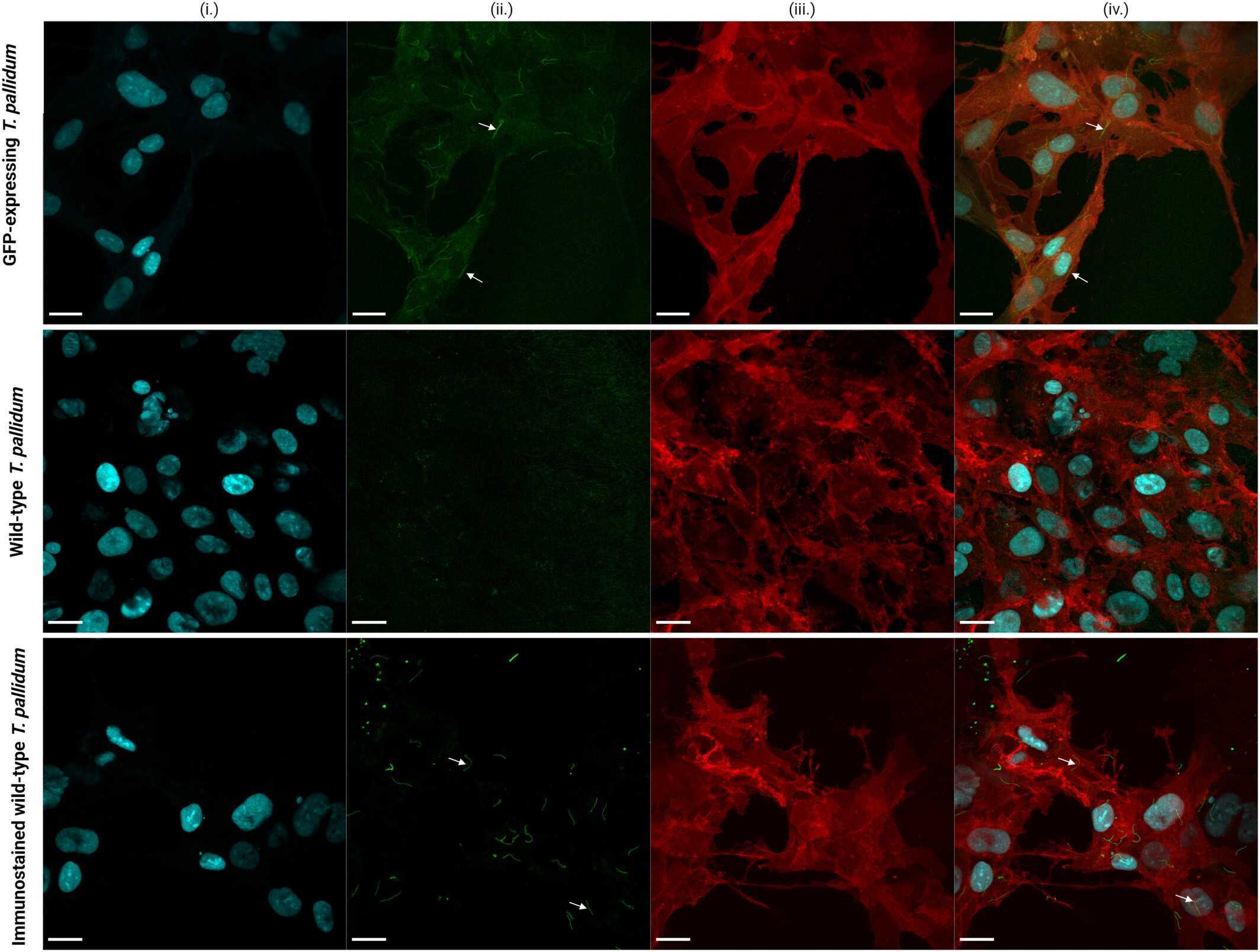
Fig1. Images of GFP-expressing Treponema pallidum and wild-type T. pallidum grown on mCherry and BFP-expressing Sf1Ep cells.
Case 2: Gaytán P, et al. ACS Omega. 2024
Photoactivatable and photoswitchable fluorescent proteins (FPs) serve as advanced molecular tools. When used in conjunction with super-resolution microscopy, they aid in clarifying numerous biological processes. By introducing the Y66H mutation into the chromophore of the violet fluorescent protein SumireF, researchers developed the inaugural photoactivatable blue fluorescent protein (PA-BFP). This protein can be swiftly activated using standard UV transilluminators at 302 or 365 nm in an irreversible manner, or by direct exposure to sunlight. The peak excitation and emission wavelengths for this protein are 358 and 445 nm, respectively. These values align closely with those of DAPI-a blue stain extensively utilized in fluorescence microscopy for visualizing nucleic acids in cells. As a result, the immediate application of PA-BFP in cellular biology is evident, given that the technology needed to observe this novel genetically encoded marker at the microscopic level is already well-established. Moreover, PA-BFP can potentially be employed alongside other photoactivatable fluorescent proteins of varying colors. This combination could enable the labeling of multiple proteins, which could then be simultaneously monitored using sophisticated microscopic methods.
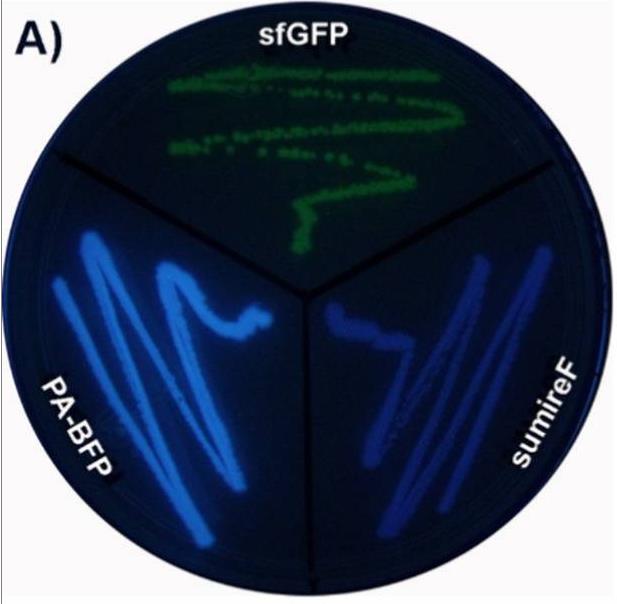
Fig1. Comparison of E. coli streaks expressing the protein PA-BFP and its precursor proteins SumireF and sfGFP.
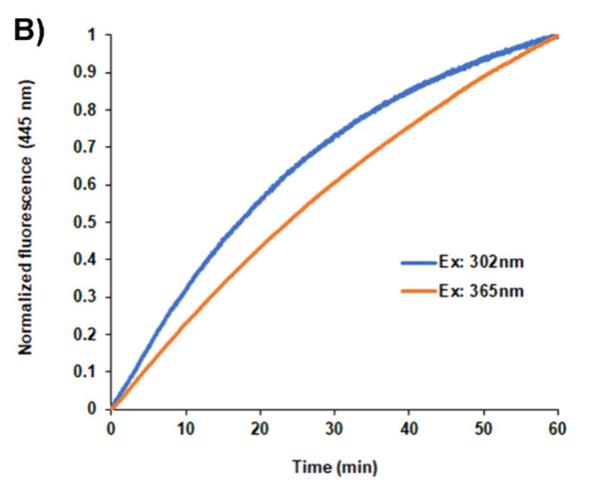
Fig2. Photoactivation reaction of the pure dark protein PA-BFP in solution.
BFPs are a class of proteins that fluoresce blue and are often used as biomarkers. Although BFPs may not be as bright and light stable as some other colored fluorescent proteins, such as enhanced Green fluorescent protein (EGFP), through genetic engineering and mutation screening, scientists have developed BFP variants with higher brightness and better light stability.
They can be used in combination with fluorescent proteins of other colors, enabling multi-label experiments that help researchers more accurately observe multiple biomolecules or organelles at the same time in the same sample. BFP is used as a donor or recipient of FRET technology to monitor protein-protein interactions and intracellular environmental changes. Compared with other intracellular luminescent proteins, BFP has the characteristics of good fluorescence stability and high sensitivity, which is suitable for the quantitative analysis of proteins. Through structural transformation, BFP can be endowed with new properties and functions, and its application in biosensing, bioimaging and other life fields can be expanded.
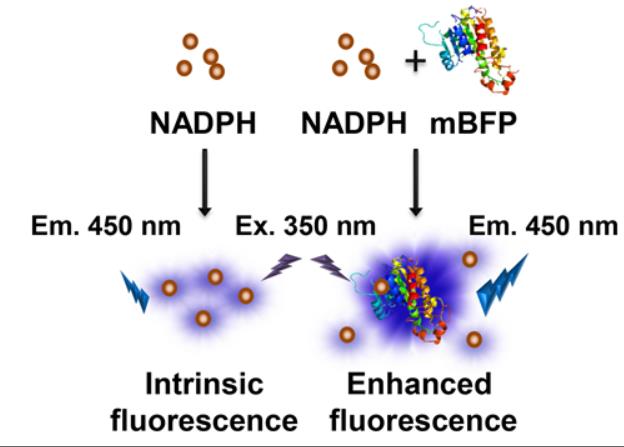
Fig1. Schematic representation of the interaction between NADPH and mBFP. (Sung-Hwan You, 2019)
Not For Human Consumption!
Inquiry
- Reviews
- Q&As
Ask a Question for All BFP Products
Required fields are marked with *
My Review for All BFP Products
Required fields are marked with *
Inquiry Basket


