TPSB2
-
Official Full Name
tryptase beta 2 (gene/pseudogene) -
Synonyms
TPSB2;tryptase beta 2 (gene/pseudogene);tryptase beta 2;tryptase beta-2;tryptase beta II;tryptase beta III;tryptase-2;tryptase II;tryptase III;mast cell tryptase beta II;mast cell tryptase beta III;TPS2;tryptaseB;tryptaseC
Recombinant Proteins
- Human
- Rat
- Mouse
- E.coli
- HEK293
- Mammalian Cells
- Human Cells
- NS0
- Yeast
- His
- T7
- Fc
- Non
- Myc
- GST
- Avi
Background
What is TPSB2 protein?
TPSB2 (tryptase beta 2) gene is a protein coding gene which situated on the short arm of chromosome 16 at locus 16p13. Tryptases comprise a family of trypsin-like serine proteases, the peptidase family S1. Tryptases are enzymatically active only as heparin-stabilized tetramers, and they are resistant to all known endogenous proteinase inhibitors. Several tryptase genes are clustered on chromosome 16p13.3. These genes are characterized by several distinct features. They have a highly conserved 3' UTR and contain tandem repeat sequences at the 5' flank and 3' UTR which are thought to play a role in regulation of the mRNA stability. These genes have an intron immediately upstream of the initiator Met codon, which separates the site of transcription initiation from protein coding sequence. This feature is characteristic of tryptases but is unusual in other genes. The alleles of this gene exhibit an unusual amount of sequence variation, such that the alleles were once thought to represent two separate genes, beta II and beta III. The TPSB2 protein is consisted of 275 amino acids and its molecular mass is approximately 30.7 kDa.
What is the function of TPSB2 protein?
The functionally related articles suggest that the TPSB2 gene is involved in proteolysis, a process where proteins are broken down into smaller polypeptides or single amino acids. The gene is expected to have peptidase activity and localize in the extracellular region, indicating its role in extracellular matrix remodeling and possibly in the activation or inactivation of other proteins through proteolytic cleavage. It is also noted that TPSB2 may be modulated by phosphorylation, which can affect its activity and interactions with other proteins. The presence of multiple spliced variants and alternative polyadenylation sites indicates a complex regulation of the gene expression.
TPSB2 Related Signaling Pathway
TPSB2 is expressed in immune cells, particularly mast cells, and plays a role in the innate immune response. It is involved in the activation and degranulation of mast cells, which release various inflammatory mediators. TPSB2 can activate PAR-2 (Protease-Activated Receptor 2), a G protein-coupled receptor that plays a role in various cellular processes, including cell proliferation, migration, and apoptosis. There is evidence suggesting that TPSB2 may be involved in tumor metastasis and angiogenesis. It can degrade extracellular matrix proteins, facilitating the spread of cancer cells, and may also play a role in the formation of new blood vessels in tumors.
TPSB2 Related Diseases
TPSB2 has been associated with various types of cancer. It is found to be overexpressed in certain cancers and may play a role in tumor growth, progression, and metastasis. It is being explored as a potential therapeutic target for cancers such as Hodgkin's lymphoma and renal cell carcinoma. TPSB2 has been suggested to play a role in neurodegenerative processes. TPSB2 is expressed in immune cells, particularly mast cells, and is involved in the innate immune response. TPSB2 has been implicated in the development of tumor-associated brain edema. It is suggested that mast cells secrete tryptase, which can induce TAH by affecting the structure of cilia in the choroid plexus.
Bioapplications of TPSB2
TPSB2 can be used as a biomarker for certain diseases, including different types of cancer. Its elevated levels in the serum can indicate the presence of disease, particularly in conditions like Hodgkin's lymphoma and renal cell carcinoma. Due to its involvement in various diseases, TPSB2 is considered a potential therapeutic target. Research into the role of TPSB2 in TAH has led to the identification of TPSB2 inhibitors, such as BMS-262084, which can ameliorate the symptoms of TAH in animal models.
Case Study
Case Study 1: Kristina M Cook, 2013
The S1A serine proteases function in many key biological processes such as development, immunity, and blood coagulation. S1A proteases contain a highly conserved disulfide bond (Cys(191)-Cys(220) in chymotrypsin numbering) that links two β-loop structures that define the rim of the active site pocket. Mast cell βII-tryptase is a S1A protease that is associated with pathological inflammation. In this study, the researchers have found that the conserved disulfide bond (Cys(220)-Cys(248) in βII-tryptase) exists in oxidized and reduced states in the enzyme stored and secreted by mast cells. The disulfide bond has a standard redox potential of -301 mV and is stoichiometrically reduced by the inflammatory mediator, thioredoxin, with a rate constant of 350 m(-1) s(-1). The oxidized and reduced enzymes have different substrate specificity and catalytic efficiency for hydrolysis of both small and macromolecular substrates.
 was incubated without or with thioredoxin.jpg)
Fig1. βII-tryptase (1 μm) was incubated without or with thioredoxin (1 μm) and then labeled with PEG-maleimide.
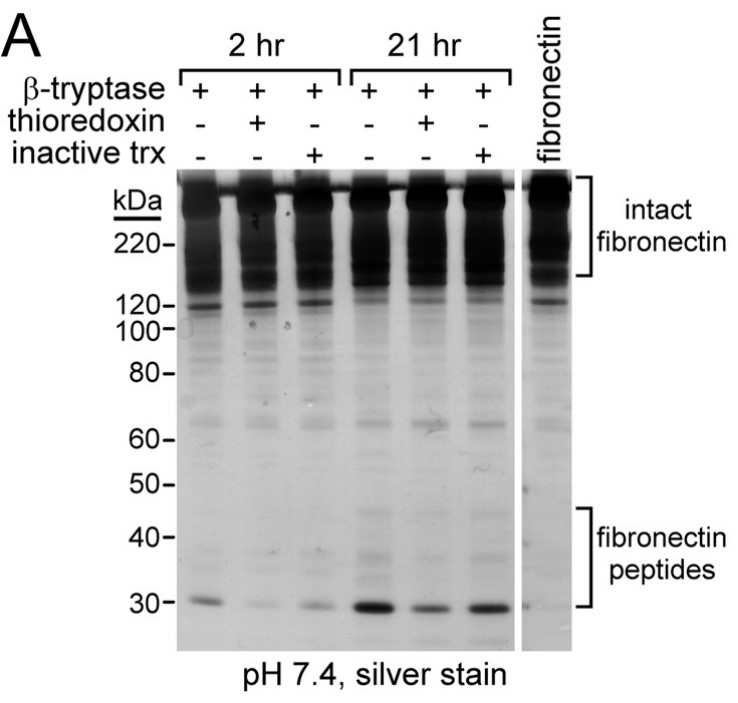
Fig2. Oxidized and reduced βII-tryptase cleave macromolecular substrates with different efficiency and specificity.
Case Study 2: Quang T Le, 2019
Both α-tryptase and β-tryptase are preferentially expressed by human mast cells, but the purpose of α-tryptase is enigmatic, because its tetramers lack protease activity, whereas β-tryptase tetramers are active proteases. The monogenic disorder called hereditary α-tryptasemia, due to increased α-tryptase gene copies and protein expression, presents with clinical features such as vibratory urticaria and dysautonomia. The researchers show that heterotetramers composed of 2α- and 2β-tryptase protomers (α/β-tryptase) form naturally in individuals who express α-tryptase. α/β-Tryptase, but not homotetramer, activates protease-activated receptor-2 (PAR2), which is expressed on cell types such as smooth muscle, neurons, and endothelium. Also, only α/β-tryptase makes mast cells susceptible to vibration-triggered degranulation by cleaving the α subunit of the EGF-like module-containing mucin-like hormone receptor-like 2 (EMR2) mechanosensory receptor.
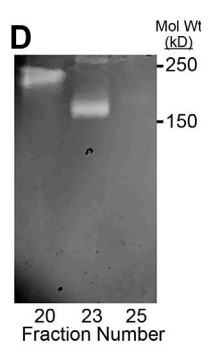
Fig3. Gelatin zymography after extended electrophoresis.
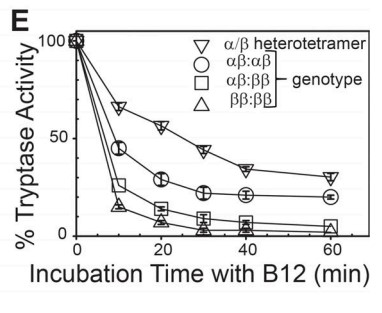
Fig4. Stability of α/β-tryptase to inhibition by B12 anti-tryptase mAb.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (TPSB2-1488H)
.
.jpg)
Fig2. SDS-PAGE (TPSB2-6279H)
Involved Pathway
TPSB2 involved in several pathways and played different roles in them. We selected most pathways TPSB2 participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with TPSB2 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
TPSB2 has several biochemical functions, for example, protein binding,serine-type endopeptidase activity,serine-type peptidase activity. Some of the functions are cooperated with other proteins, some of the functions could acted by TPSB2 itself. We selected most functions TPSB2 had, and list some proteins which have the same functions with TPSB2. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| protein binding | ZFC3H1,CHRD,ANO2,STIM1,RRM2,LGALS9C,UBL4B,NFASC,GABARAPL1,FLCN |
| serine-type endopeptidase activity | FURIN,ST14B,TPP1,OVCH2,PRSS41,KLK1B9,PCSK1,HTRA4,HP,CORIN |
| serine-type peptidase activity | TMPRSS11E,HTRA1A,DPP8,KLK11,KLK1B8,Reln,HTRA2,KLK12,TMPRSS13B,GZMK |
Interacting Protein
TPSB2 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with TPSB2 here. Most of them are supplied by our site. Hope this information will be useful for your research of TPSB2.
NCK1;PIK3R1
Resources
Related Services
Related Products
References
- Cui, Y; Dahlin, JS; et al. Mouse Mast Cell Protease-6 and MHC Are Involved in the Development of Experimental Asthma. JOURNAL OF IMMUNOLOGY 193:4783-4789(2014).
- Rahman, SU; Arenas, J; et al. The Polypeptide Transport-associated (POTRA) Domains of TpsB Transporters Determine the System Specificity of Two-partner Secretion Systems. JOURNAL OF BIOLOGICAL CHEMISTRY 289:19799-19809(2014).


